Label: ONDANSETRON HYDROCHLORIDE AND DEXTROSE injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0143-9771-06 - Packager: West-ward Pharmaceutical Corp
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 2, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The active ingredient in Ondansetron in 5 % Dextrose Injection is ondansetron hydrochloride (HCl), the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
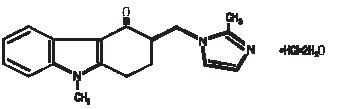
The empirical formula is C18H19N3O·HCl·2H2O, representing a molecular weight of 365.9.
Ondansetron HCl is a white to off-white powder that is soluble in water and normal saline.
Sterile, Premixed Solution for Intravenous Administration in Single-Dose, Flexible Plastic Containers: Each 50 mL contains ondansetron 32 mg (as the hydrochloride dihydrate); dextrose 2500 mg; and citric acid 26 mg and sodium citrate 11.5 mg as buffers in Water for Injection, USP.
It contains no preservatives. The osmolarity of this solution is 270 mOsm/L (approx.), and the pH is 3 to 4.
The flexible plastic container is fabricated from a specially formulated, plastic film. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics:
Ondansetron is a selective 5-HT3 receptor antagonist. While ondansetron's mechanism of action has not been fully characterized, it is not a dopamine-receptor antagonist. Serotonin receptors of the 5-HT3 type are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. It is not certain whether ondansetron's antiemetic action in chemotherapy-induced nausea and vomiting is mediated centrally, peripherally, or in both sites. However, cytotoxic chemotherapy appears to be associated with release of serotonin from the enterochromaffin cells of the small intestine. In humans, urinary 5-HIAA (5-hydroxyindoleacetic acid) excretion increases after cisplatin administration in parallel with the onset of vomiting. The released serotonin may stimulate the vagal afferents through the 5-HT3 receptors and initiate the vomiting reflex.
In animals, the emetic response to cisplatin can be prevented by pretreatment with an inhibitor of serotonin synthesis, bilateral abdominal vagotomy and greater splanchnic nerve section, or pretreatment with a serotonin 5-HT3 receptor antagonist.
In normal volunteers, single I.V. doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time. In another study in six normal male volunteers, a 16-mg dose infused over 5 minutes showed no effect of the drug on cardiac output, heart rate, stroke volume, blood pressure, or electrocardiogram (ECG). Multiday administration of ondansetron has been shown to slow colonic transit in normal volunteers. Ondansetron has no effect on plasma prolactin concentrations.
In a gender-balanced pharmacodynamic study (n = 56), ondansetron 4 mg administered intravenously or intramuscularly was dynamically similar in the prevention of nausea and vomiting using the ipecacuanha model of emesis.
Ondansetron does not alter the respiratory depressant effects produced by alfentanil or the degree of neuromuscular blockade produced by atracurium. Interactions with general or local anesthetics have not been studied.
Pharmacokinetics:
Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by glucuronide or sulfate conjugation.
Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron.
In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P-450 enzymes, including CYP1A2,
CYP2D6, and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g., CYP2D6 genetic deficiency) will be compensated by others and may result in little change in overall rates of ondansetron elimination. Ondansetron elimination may be affected by cytochrome P-450 inducers. In a pharmacokinetic study of 16 epileptic patients maintained chronically on CYP3A4 inducers, carbamazepine, or phenytoin, reduction in AUC, Cmax, and T1/2 of ondansetron was observed.1 This resulted in a significant increase in clearance. However, on the basis of available data, no dosage adjustment for ondansetron is recommended (see PRECAUTIONS: Drug Interactions).
In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
In normal adult volunteers, the following mean pharmacokinetic data have been determined following a single 0.15-mg/kg I.V. dose.
Table 1. Pharmacokinetics in Normal Adult Volunteers Age-group (years) n Peak Plasma Concentration (ng/mL) Mean Elimination Half-life (h) Plasma Clearance (L/h/kg) 19 – 40 11 102 3.5 0.381 61 – 74 12 106 4.7 0.319 ≥ 75 11 170 5.5 0.262 A reduction in clearance and increase in elimination half-life are seen in patients over 75 years of age. In clinical trials with cancer patients, safety and efficacy were similar in patients over 65 years of age and those under 65 years of age; there was an insufficient number of patients over 75 years of age to permit conclusions in that age-group. No dosage adjustment is recommended in the elderly.
In patients with mild-to-moderate hepatic impairment, clearance is reduced 2-fold and mean half-life is increased to 11.6 hours compared to 5.7 hours in normals. In patients with severe hepatic impairment (Child-Pugh2 score of 10 or greater), clearance is reduced 2-fold to 3-fold and apparent volume of distribution is increased with a resultant increase in half-life to 20 hours. In patients with severe hepatic impairment, a total daily dose of 8 mg should not be exceeded.
Due to the very small contribution (5%) of renal clearance to the overall clearance, renal impairment was not expected to significantly influence the total clearance of ondansetron. However, ondansetron mean plasma clearance was reduced by about 41% in patients with severe renal impairment (creatinine clearance <30 mL/min). This reduction in clearance is variable and was not consistent with an increase in half-life. No reduction in dose or dosing frequency in these patients is warranted.
In adult cancer patients, the mean elimination half-life was 4 hours, and there was no difference in the multidose pharmacokinetics over a 4-day period. In a study of 21 pediatric cancer patients (4 to 18 years of age) who received three I.V. doses of 0.15 mg/kg of ondansetron at 4-hour intervals, patients older than 15 years of age exhibited ondansetron pharmacokinetic parameters similar to those of adults. Patients 4 to 12 years of age generally showed higher clearance and somewhat larger volume of distribution than adults. Most pediatric patients younger than 15 years of age with cancer had a shorter (2.4 hours) ondansetron plasma half-life than patients older than 15 years of age. It is not known whether these differences in ondansetron plasma half-life may result in differences in efficacy between adults and some young pediatric patients (see CLINICAL TRIALS: Pediatric Studies).
Pharmacokinetic information for pediatric cancer patients 6 months to 48 months of age is approved for GlaxoSmithKline Corporation’s ondansetron injection. However, due to GlaxoSmithKline’s marketing exclusivity rights, this drug product is not labeled for use in this subpopulation of pediatric patients.
In a study of 21 pediatric patients (3 to 12 years of age) who were undergoing surgery requiring anesthesia for a duration of 45 minutes to 2 hours, a single I.V. dose of ondansetron, 2 mg (3 to 7 years) or 4 mg (8 to 12 years), was administered immediately prior to anesthesia induction. Mean weight-normalized clearance and volume of distribution values in these pediatric surgical patients were similar to those previously reported for young adults. Mean terminal half-life was slightly reduced in pediatric patients (range, 2.5 to 3 hours) in comparison with adults (range, 3 to 3.5 hours).
Table 2. Pharmacokinetics in Pediatric Surgery Patients 1 Month to 12 Years of Age Subjects and Age Group N CL (L/h/kg) VSS (L/kg) T 1/2 (h) Geometric Mean Mean Pediatric Surgery Patients 3 to 12 years of Age N = 21 0.439 1.65 2.9 Pediatric Surgery Patients 5 to 24 months of Age N = 22 0.581 2.3 2.9 Pediatric Surgery Patients 1 month to 4 months of Age N = 19 0.401 3.5 6.7 In general, surgical and cancer pediatric patients younger than 18 years tend to have a higher ondansetron clearance compared to adults leading to a shorter half-life in most pediatric patients. In patients 1 month to 4 months of age, a longer half-life was observed due to the higher volume of distribution in this age group.
Pharmacokinetic information for pediatric surgical patients 1 month to 24 months of age is approved for GlaxoSmithKline Corporation’s ondansetron injection. However, due to GlaxoSmithKline’s marketing exclusivity rights, this drug product is not labeled for use in this subpopulation of pediatric patients.
In normal volunteers (19 to 39 years old, n = 23), the peak plasma concentration was 264 ng/mL following a single 32-mg dose administered as a 15-minute I.V. infusion. The mean elimination half-life was 4.1 hours. Systemic exposure to 32 mg of ondansetron was not proportional to dose as measured by comparing dose-normalized AUC values to an 8-mg dose. This is consistent with a small decrease in systemic clearance with increasing plasma concentrations.
A study was performed in normal volunteers (n = 56) to evaluate the pharmacokinetics of a single 4-mg dose administered as a 5-minute infusion compared to a single intramuscular injection. Systemic exposure as measured by mean AUC was equivalent, with values of 156 [95% CI 136, 180] and 161 [95% CI 137, 190] ng•h/mL for I.V. and I.M. groups, respectively. Mean peak plasma concentrations were 42.9 [95% CI 33.8, 54.4] ng/mL at 10 minutes after I.V. infusion and 31.9 [95% CI 26.3, 38.6] ng/mL at 41 minutes after I.M. injection. The mean elimination half-life was not affected by route of administration.
Plasma protein binding of ondansetron as measured in vitro was 70% to 76%, with binding constant over the pharmacologic concentration range (10 to 500 ng/mL). Circulating drug also distributes into erythrocytes.
A positive lymphoblast transformation test to ondansetron has been reported, which suggests immunologic sensitivity to ondansetron.
-
CLINICAL TRIALS
Chemotherapy-Induced Nausea and Vomiting:
Adult Studies: In a double-blind study of three different dosing regimens of ondansetron injection, 0.015 mg/kg, 0.15 mg/kg, and 0.30 mg/kg, each given three times during the course of cancer chemotherapy, the 0.15-mg/kg dosing regimen was more effective than the 0.015-mg/kg dosing regimen. The 0.30-mg/kg dosing regimen was not shown to be more effective than the 0.15-mg/kg dosing regimen.
Cisplatin-Based Chemotherapy: In a double-blind study in 28 patients, ondansetron injection (three 0.15-mg/kg doses) was significantly more effective than placebo in preventing nausea and vomiting induced by cisplatin-based chemotherapy. Treatment response was as shown in Table 3.
Table 3. Prevention of Chemotherapy-Induced Nausea and Vomiting in Single-Day Cisplatin Therapy* in Adults Ondansetron Injection Placebo P Value † Number of patients 14 14 Treatment response
0 Emetic episodes
1-2 Emetic episodes
3-5 Emetic episodes
More than 5 emetic episodes/rescued2 (14%)
8 (57%)
2 (14%)
2 (14%)0 (0%)
0 (0%)
1 (7%)
13 (93%)0.001 Median number of emetic episodes 1.5 Undefined‡ Median time to first emetic episode (h) 11.6 2.8 0.001 Median nausea scores (0 – 100) § 3 59 0.034 Global satisfaction with control of nausea and vomiting (0 – 100) II 96 1
0.50.009 * Chemotherapy was high dose (100 and 120 mg/m2 ; Ondansetron injection n = 6, placebo n = 5) or moderate dose (50 and 80 mg/m2; Ondansetron injection n = 8, placebo n = 9). Other chemotherapeutic agents included fluorouracil, doxorubicin, and cyclophosphamide.
There was no difference between treatments in the types of chemotherapy that would account for differences
in response.† Efficacy based on "all patients treated" analysis.
‡ Median undefined since at least 50% of the patients were rescued or had more than five emetic episodes.
§ Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be.
II Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied.
Ondansetron was compared with metoclopramide in a single-blind trial in 307 patients receiving cisplatin >100 mg/m2 with or without other chemotherapeutic agents. Patients received the first dose of ondansetron or metoclopramide 30 minutes before cisplatin. Two additional ondansetron doses were administered 4 and 8 hours later, or five additional metoclopramide doses were administered 2, 4, 7, 10, and 13 hours later. Cisplatin was administered over a period of 3 hours or less. Episodes of vomiting and retching were tabulated over the period of 24 hours after cisplatin. The results of this study are summarized in Table 4.
Table 4. Prevention of Vomiting Induced by Cisplatin (>100 mg/m2) Single-Day Therapy* in Adults Ondansetron
InjectionMetoclopramide P Value Dose 0.15 mg/kg x 3 2mg/kg x 6 Number of patients in efficacy population 136 Treatment response
0 Emetic episodes
1-2 Emetic episodes
3-5 Emetic episodes
More than 5 emetic episodes/rescued54 (40%)
34 (25%)
19 (14%)
29 (21%)41 (30%)
30 (22%)
18 (13%)
49 (36%)Comparison of treatments with respect to
0 Emetic episodes
More than 5 emetic episodes/rescued54/136
29/13641/138
49/1380.083
0.009Median number of emetic episodes 1 2 0.005 Median time to first emetic episode (h) 20.5 4.3 <0.001 Global satisfaction with control of nausea and vomiting (0 – 100) † 85 63 0.001 Acute dystonic reactions 0 8 0.005 Akathisia 0 10 0.002 * In addition to cisplatin, 68% of patients received other chemotherapeutic agents, including cyclophosphamide,
etoposide, and fluorouracil. There was no difference between treatments in the types of chemotherapy that would account for differences in response.† Visual analog scale assessment: 0 = not at all satisfied, 100 = totally satisfied.
In a stratified, randomized, double-blind, parallel-group, multicenter study, a single 32-mg dose of ondansetron was compared with three 0.15-mg/kg doses in patients receiving cisplatin doses of either 50 to 70 mg/m2 or >100 mg/m2. Patients received the first ondansetron dose 30 minutes before cisplatin. Two additional ondansetron doses were administered 4 and 8 hours later to the group receiving three 0.15-mg/kg doses. In both strata, significantly fewer patients on the single 32-mg dose than those receiving the three-dose regimen failed.
Table 5. Prevention of Chemotherapy-Induced Nausea and Vomiting in Single-Dose Therapy in Adults 0.15mg/kg x 3 Ondansetron Dose
32mg x 1P Value High-dose cisplatin (≥ 100mg/m2)
Number of patients100 102 Treatment response
0 Emetic episodes
1-2 Emetic episodes
3-5 Emetic episodes
More than 5 emetic episodes/rescued41 (41%)
19 (19%)
4 (4%)
36 (36%)49 (48%)
25 (25%)
8 (8%)
20 (20%)0.315
0.009Median time to first emetic episode (h) 21.7 23 0.173 Median nausea scores (0 – 100)* 28 13 0.004 Medium-dose cisplatin (50 – 70mg/m2) Number of patients 101 93 Treatment response
0 Emetic episodes
1 -2 Emetic episodes
3 – 5 Emetic episodes
More than 5 emetic episodes/rescued62 (61%)
11 (11%)
6 (6%)
22 (22%)69 (73%)
14 (15%)
3 (3%)
8 (9%)0.083
0.011Median time to first emetic episode (h) Undefined† Undefined Median nausea scores (0 – 100)* 0 3 0.131 * Visual analog scale assessment: 0 = no nausea, 100 = nausea as bad as it can be.
† Median undefined since at least 50% of patients did not have any emetic episodes.
Cyclophosphamide-Based Chemotherapy: In a double-blind, placebo-controlled study of ondansetron injection (three 0.15-mg/kg doses) in 20 patients receiving cyclophosphamide (500 to 600 mg/m2) chemotherapy, ondansetron injection was significantly more effective than placebo in preventing nausea and vomiting. The results are summarized in Table 6.
Table 6. Prevention of Chemotherapy-Induced Nausea and Vomiting in Single-Day Cyclophosphamide Therapy* in Adults Ondansetron Injection Placebo P Value ‡ Number of patients 10 10 Treatment response
0 Emetic episodes
1-2 Emetic episodes
3-5 Emetic episodes
More than 5 emetic episodes/rescued7 (70%)
0 (0%)
2 (20%)
1 (10%)0 (0%)
2 (20%)
4 (40%)
4 (40%)0.001
0.131Median number of emetic episodes 0 4 0.008 Median time to first emetic episode (h) Undefined‡ 8.79 Median nausea scores (0 – 100) § 0 60 0.001 Global satisfaction with control of nausea and vomiting (0 – 100) II 100 52 0.008 * Chemotherapy consisted of cyclophosphamide in all patients, plus other agents, including fluorouracil, doxorubicin, methotrexate, and vincristine. There was no difference between treatments in the type of chemotherapy that would account for differences in response.
† Efficacy based on "all patients treated" analysis.
‡ Median undefined since at least 50% of patients did not have any emetic episodes.
§ Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be.
II Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied.
Re-treatment: In uncontrolled trials, 127 patients receiving cisplatin (median dose, 100 mg/m2) and ondansetron who had two or fewer emetic episodes were re-treated with ondansetron and chemotherapy, mainly cisplatin, for a total of 269 re-treatment courses (median, 2; range, 1 to 10). No emetic episodes occurred in 160 (59%), and two or fewer emetic episodes occurred in 217 (81%) re-treatment courses.
Pediatric Studies:
Four open-label, noncomparative (one US, three foreign) trials have been performed with 209 pediatric cancer patients 4 to 18 years of age given a variety of cisplatin or noncisplatin regimens. In the three foreign trials, the initial ondansetron injection dose ranged from 0.04 to 0.87 mg/kg for a total dose of 2.16 to 12 mg. This was followed by the oral administration of ondansetron ranging from 4 to 24 mg daily for 3 days. In the US trial, ondansetron injection was administered intravenously (only) in three doses of 0.15 mg/kg each for a total daily dose of 7.2 to 39 mg. In these studies, 58% of the 196 evaluable patients had a complete response (no emetic episodes) on day 1. Thus, prevention of vomiting in these pediatric patients was essentially the same as for patients older than 18 years of age.
Clinical trial information in pediatric cancer patients 6 months to 48 months of age is approved for GlaxoSmithKline Corporation’s ondansetron injection. However, due to GlaxoSmithKline’s marketing exclusivity rights, this drug product is not labeled for use in this subpopulation of pediatric patients.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention.
Rarely and predominantly with intravenous ondansetron, transient ECG changes including QT interval prolongation have been reported.
Drug Interactions:
Ondansetron does not itself appear to induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system of the liver (see CLINICAL PHARMACOLOGY: Pharmacokinetics). Because ondansetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes (CYP3A4, CYP2D6, CYP1A2), inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of ondansetron. On the basis of limited available data, no dosage adjustment is recommended for patients on these drugs.
Phenytoin, Carbamazepine, and Rifampicin:
In patients treated with potent inducers of CYP3A4 (i.e., phenytoin, carbamazepine, and rifampicin), the clearance of ondansetron was significantly increased and ondansetron blood concentrations were decreased. However, on the basis of available data, no dosage adjustment for ondansetron is recommended for patients on these drugs.1,3
Tramadol:
Although no pharmacokinetic drug interaction between ondansetron and tramadol has been observed, data from 2 small studies indicate that ondansetron may be associated with an increase in patient controlled administration of tramadol.4,5
Chemotherapy:
Tumor response to chemotherapy in the P 388 mouse leukemia model is not affected by ondansetron. In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
In a crossover study in 76 pediatric patients, I.V. ondansetron did not increase blood levels of high-dose methotrexate.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 and 30 mg/kg per day, respectively. Ondansetron was not mutagenic in standard tests for mutagenicity. Oral administration of ondansetron up to 15 mg/kg per day did not affect fertility or general reproductive performance of male and female rats.
Pregnancy:
Teratogenic Effects:
Pregnancy Category B. Reproduction studies have been performed in pregnant rats and rabbits at I.V. doses up to 4 mg/kg per day and have revealed no evidence of impaired fertility or harm to the fetus due to ondansetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers:
Ondansetron is excreted in the breast milk of rats. It is not known whether ondansetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ondansetron is administered to a nursing woman.
Pediatric Use:
Little information is available about the use of ondansetron in pediatric surgical patients younger than 1 month of age. Little information is available about the use of ondansetron in pediatric cancer patients younger than 6 months of age. The clearance of ondansetron in pediatric patients 1 month to 4 months of age is slower and the half-life is ~2.5 fold longer than patients who are >4 to 24 months of age. As a precaution, it is recommended that patients less than 4 months of age receiving this drug be closely monitored. (See CLINICAL PHARMACOLOGY: Pharmacokinetics).
The frequency and type of adverse events reported in pediatric patients receiving ondansetron were similar to those in patients receiving placebo.
Geriatric Use:
Of the total number of subjects enrolled in cancer chemotherapy-induced and postoperative nausea and vomiting in US- and foreign-controlled clinical trials, 862 were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment is not needed in patients over the age of 65 (see CLINICAL PHARMACOLOGY).
-
ADVERSE REACTIONS
Chemotherapy-Induced Nausea and Vomiting:
The adverse events in Table 7 have been reported in adults receiving ondansetron at a dosage of three 0.15-mg/kg doses or as a single 32-mg dose in clinical trials. These patients were receiving concomitant chemotherapy, primarily cisplatin, and I.V. fluids. Most were receiving a diuretic.
Table 7. Principal Adverse Events in Comparative Trials in Adults Number of Adult Patients With Event Ondansetron Injection, USP
0.15mg/kg x 3
N = 419Ondansetron Injection, USP
32mg x 1
N = 220Metoclopramide
N = 156Placebo
N = 34Diarrhea 16% 8% 44% 18% Headache 17% 25% 7% 15% Fever 8% 7% 5% 3% Akathisia 0% 0% 6% 0% Acute dystonic reactions* 0% 0% 5% 0% *See Neurological
The following have been reported during controlled clinical trials:
Cardiovascular: Rare cases of angina (chest pain), electrocardiographic alterations, hypotension, and tachycardia have been reported. In many cases, the relationship to ondansetron injection was unclear.
Gastrointestinal: Constipation has been reported in 11% of chemotherapy patients receiving multiday ondansetron.
Hepatic: In comparative trials in cisplatin chemotherapy patients with normal baseline values of aspartate transaminase (AST) and alanine transaminase (ALT), these enzymes have been reported to exceed twice the upper limit of normal in approximately 5% of patients. The increases were transient and did not appear to be related to dose or duration of therapy. On repeat exposure, similar transient elevations in transaminase values occurred in some courses, but symptomatic hepatic disease did not occur.
Integumentary: Rash has occurred in approximately 1% of patients receiving ondansetron.
Neurological: There have been rare reports consistent with, but not diagnostic of, extrapyramidal reactions in patients receiving ondansetron injection, and rare cases of grand mal seizure. The relationship to ondansetron injection was unclear.
Other: Rare cases of hypokalemia have been reported. The relationship to ondansetron injection was unclear.
Observed During Clinical Practice: In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of intravenous formulations of ondansetron injection. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to ondansetron injection.
Cardiovascular: Arrhythmias (including ventricular and supraventricular tachycardia, premature ventricular contractions, and atrial fibrillation), bradycardia, electrocardiographic alterations (including second-degree heart block, QT interval prolongation and ST segment depression), palpitations, and syncope.
General: Flushing. Rare cases of hypersensitivity reactions, sometimes severe (e.g., anaphylaxis/anaphylactoid reactions, angioedema, bronchospasm, cardiopulmonary arrest, hypotension, laryngeal edema, laryngospasm, shock, shortness of breath, stridor) have also been reported.
Hepatobiliary: Liver enzyme abnormalities have been reported. Liver failure and death have been reported in patients with cancer receiving concurrent medications including potentially hepatotoxic cytotoxic chemotherapy and antibiotics. The etiology of the liver failure is unclear.
Local Reactions: Pain, redness, and burning at site of injection.
Lower Respiratory: Hiccups
Neurological: Oculogyric crisis, appearing alone, as well as with other dystonic reactions.
Skin: Urticaria
Special Senses: Transient dizziness during or shortly after I.V. infusion.
Eye Disorders: Transient blurred vision, in some cases associated with abnormalities of accommodation. Cases of transient blindness, predominantly during intravenous administration, have been reported. These cases of transient blindness were reported to resolve within a few minutes up to 48 hours.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual doses as large as 150 mg and total daily dosages (three doses) as large as 252 mg have been administered intravenously without significant adverse events. These doses are more than 10 times the recommended daily dose.
In addition to the adverse events listed above, the following events have been described in the setting of ondansetron overdose: "Sudden blindness" (amaurosis) of 2 to 3 minutes' duration plus severe constipation occurred in one patient that was administered 72 mg of ondansetron intravenously as a single dose. Hypotension (and faintness) occurred in another patient that took 48 mg of oral ondansetron. Following infusion of 32 mg over only a 4-minute period, a vasovagal episode with transient second-degree heart block was observed. In all instances, the events resolved completely.
-
DOSAGE AND ADMINISTRATION
Prevention of Chemotherapy-Induced Nausea and Vomiting:
Adult Dosing:
The recommended I.V. dosage of ondansetron injection for adults is a single 32-mg dose or three 0.15-mg/kg doses. A single 32-mg dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. The recommended infusion rate should not be exceeded (see OVERDOSAGE). With the three-dose (0.15-mg/kg) regimen, the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. Subsequent doses (0.15 mg/kg) are administered 4 and 8 hours after the first dose of ondansetron injection.
Ondansetron injection should not be mixed with solutions for which physical and chemical compatibility has not been established. In particular, this applies to alkaline solutions as a precipitate may form.
Flexible Plastic Container: REQUIRES NO DILUTION. Ondansetron in 5 % Dextrose Injection, 32 mg in 50 mL.
Pediatric Dosing:
On the basis of the available information (see CLINICAL TRIALS: Pediatric Studies and CLINICAL PHARMACOLOGY: Pharmacokinetics), the dosage in pediatric cancer patients 4 to 18 years of age should be three 0.15-mg/kg doses. The first dose is to be administered 30 minutes before the start of moderately to highly emetogenic chemotherapy, subsequent doses (0.15 mg/kg) are administered 4 and 8 hours after the first dose of Ondansetron in 5 % Dextrose Injection. The drug should be infused intravenously over 15 minutes. Little information is available about dosage in pediatric cancer patients younger than 6 months of age.
Dosing information for pediatric cancer patients 6 months to 48 months of age is approved for GlaxoSmithKline Corporation’s ondansetron injection. However, due to GlaxoSmithKline’s marketing exclusivity rights, this drug product is not labeled for use in this subpopulation of pediatric patients.
Flexible Plastic Container: REQUIRES NO DILUTION. Ondansetron in 5 % Dextrose Injection, 32 mg in 50 mL.
Dosage Adjustment for Patients With Impaired Renal Function:
The dosage recommendation is the same as for the general population. There is no experience beyond first-day administration of ondansetron.
Dosage Adjustment for Patients With Impaired Hepatic Function:
In patients with severe hepatic impairment (Child-Pugh2 score of 10 or greater), a single maximal daily dose of 8 mg to be infused over 15 minutes beginning 30 minutes before the start of the emetogenic chemotherapy is recommended. There is no experience beyond first-day administration of ondansetron.
Ondansetron in 5 % Dextrose Injection in Flexible Plastic Containers: Instructions for Use:
To Open: Tear outer wrap at notch and remove solution container. Check for minute leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
Preparation for Administration: Use aseptic technique.
1. Close flow control clamp of administration set.
2. Remove cover from outlet port at bottom of container.
3. Insert piercing pin of administration set into port with a twisting motion until the pin
is firmly seated. NOTE: See full directions on administration set carton.
4. Suspend container from hanger.
5. Squeeze and release drip chamber to establish proper fluid level in chamber during
infusion of Ondansetron in 5 % Dextrose Injection.
6. Open flow control clamp to expel air from set. Close clamp.
7. Attach set to venipuncture device. If device is not indwelling, prime and make
venipuncture.
8. Perform venipuncture.
9. Regulate rate of administration with flow control clamp.
Caution: Ondansetron in 5 % Dextrose Injection in flexible plastic containers is to be administered by I.V. drip infusion only. Ondansetron in 5 % Dextrose Injection should not be mixed with solutions for which physical and chemical compatibility have not been established. In particular, this applies to alkaline solutions as a precipitate may form. If used with a primary I.V. fluid system, the primary solution should be discontinued during ondansetron in 5 % Dextrose Injection. Do not administer unless solution is clear and container is undamaged.
Warning: Do not use flexible plastic container in series connections.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit.
-
HOW SUPPLIED
Ondansetron in 5 % Dextrose Injection, 32 mg/50 mL, in 5% Dextrose, contains no preservatives and is supplied as a sterile, premixed solution for I.V. administration in single-dose, flexible plastic containers (NDC 0143-9771-06) (case of 6).
Store between 20° and 25°C (68° and 77°F). [See USP Controlled Room Temperature] Protect from light. Avoid excessive heat. Protect from freezing.
-
REFERENCES
- Britto MR, Hussey EK, Mydlow P, et al. Effect of enzyme inducers on ondansetron (OND) metabolism in humans. Clin Pharmacol Ther. 1997;61:228.
- Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. BritJSurg. 1973;60:646-649.
- Villikka K, Kivisto KT, Neuvonen PJ. The effect of rifampin on the pharmacokinetics of oral and intravenous ondansetron. Clin Pharmacol Ther. 1999;65:377-381.
- De Witte JL, Schoenmaekers B, Sessler DI, et al. Anesth Analg. 2001;92:1319-1321.
- Arcioni R, della Rocca M, Romanò R, et al. Anesth Analg. 2002;94:1553-1557.
Rx Only
Manufactured by:
Claris Lifesciences Ltd. IndiaDistributed by:
West-ward Injectables
Eatontown, NJ 07724 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONDANSETRON HYDROCHLORIDE AND DEXTROSE
ondansetron hydrochloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0143-9771 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONDANSETRON HYDROCHLORIDE (UNII: NMH84OZK2B) (ONDANSETRON - UNII:4AF302ESOS) ONDANSETRON HYDROCHLORIDE 32 mg in 50 mL Inactive Ingredients Ingredient Name Strength DEXTROSE (UNII: IY9XDZ35W2) 2.5 g in 50 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 26 mg in 50 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 11.5 mg in 50 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0143-9771-06 6 in 1 CASE 1 50 mL in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078308 08/04/2009 Labeler - West-ward Pharmaceutical Corp (001230762)


