TRIPLE ANTIBIOTIC- bacitracin, neomycin, polymyxin b ointment
Perrigo New York Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Perrigo Triple Antibiotic Ointment Drug Facts
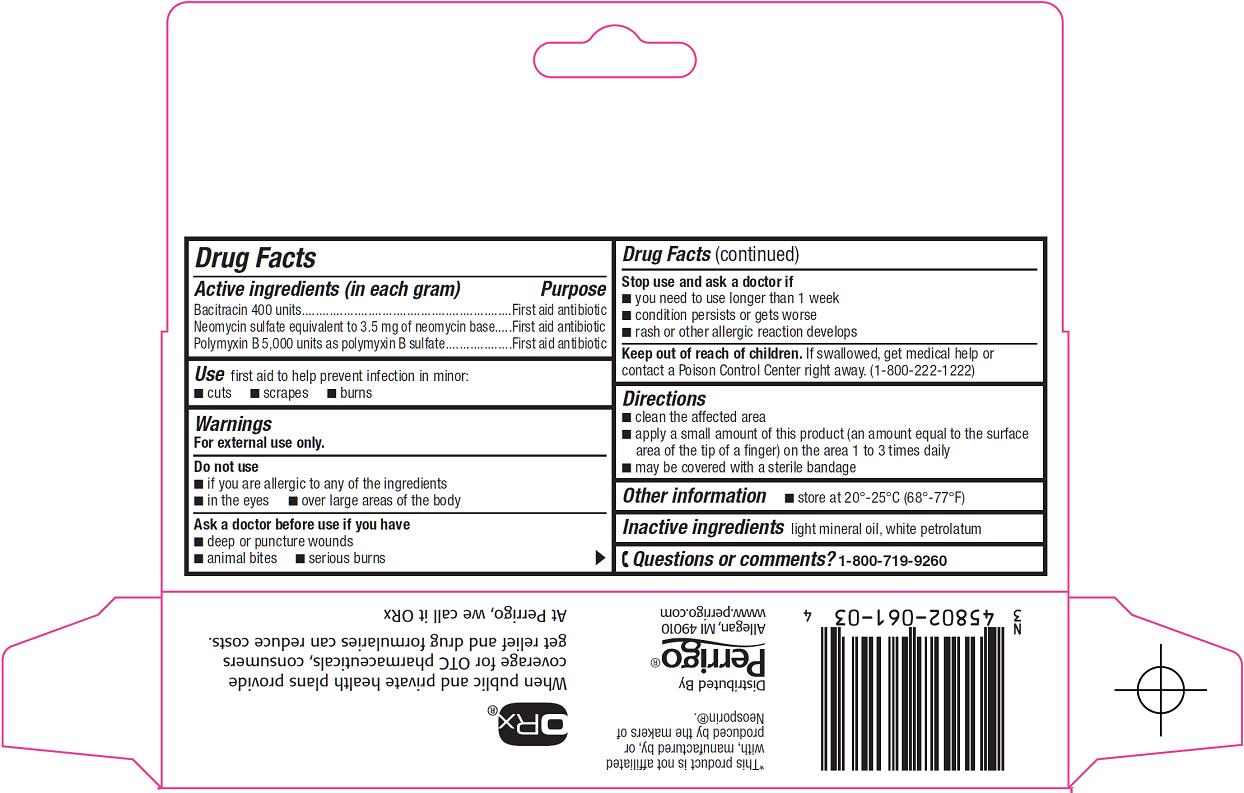
Active ingredient (in each gram)
Bacitracin 400 units
Neomycin sulfate equivalent to 3.5 mg of neomycin base
Polymyxin B 5,000 units as polymyxin B sulfate
Warnings
For external use only.
Do not use
- •
- if you are allergic to any of the ingredients
- •
- in the eyes
- •
- over large areas of the body
| TRIPLE ANTIBIOTIC
bacitracin, neomycin, polymyxin b ointment |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Perrigo New York Inc (078846912) |
Revised: 12/2018
Document Id: 7a40e301-63f8-485e-9d5a-9e7f56beb866
Set id: 675ae798-d015-4e98-a7c3-2c6f33e7d260
Version: 3
Effective Time: 20181221
Perrigo New York Inc