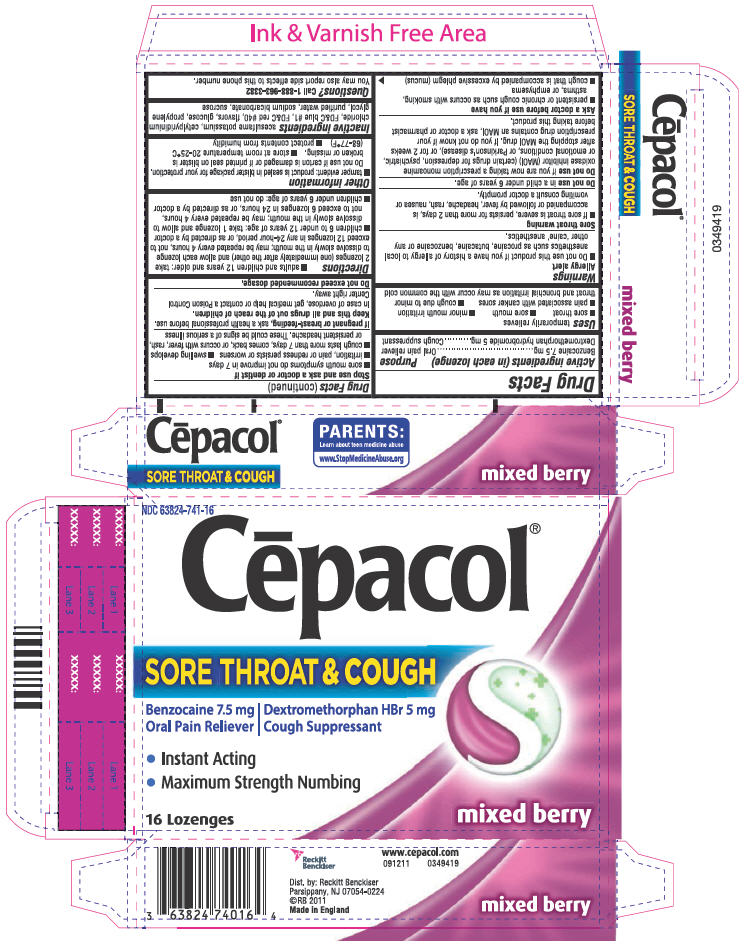
CEPACOL SORE THROAT AND COUGH- benzocaine and dextromethorphan hydrobromide lozenge
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cēpacol
®
SORE THROAT AND COUGH
Uses
temporarily relieves
- sore throat
- sore mouth
- minor mouth irritation
- pain associated with canker sores
- cough due to minor throat and bronchial irritation as may occur with the common cold
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that is accompanied by excessive phlegm (mucus)
Do not exceed recommended dosage.
Directions
- adults and children 12 years and older: take 2 lozenges (one immediately after the other) and allow each lozenge to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 12 lozenges in any 24-hour period, or as directed by a doctor
- children 6 to under 12 years of age: take 1 lozenge and allow to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 6 lozenges in 24 hours, or as directed by a doctor
- children under 6 years of age: do not use
Other information
- tamper evident: product is sealed in blister package for your protection. Do not use if carton is damaged or if printed seal on blister is broken or missing.
- store at room temperature 20-25°C (68-77°F)
- protect contents from humidity
| CEPACOL
SORE THROAT AND COUGH
benzocaine and dextromethorphan hydrobromide lozenge |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Revised: 7/2023
Document Id: 017f7614-fff1-5264-e063-6294a90a382e
Set id: 66b43243-f29e-4e8c-925d-692d894de240
Version: 2
Effective Time: 20230727
RB Health (US) LLC