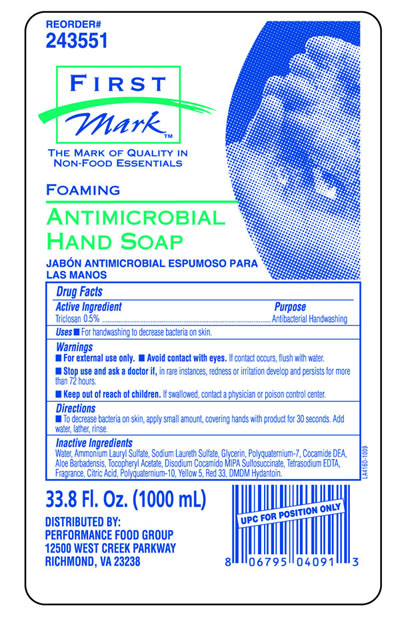
FIRST MARK FOAM ANTIMICROBIAL HAND CLEANER- triclosan liquid
Performance Food Group
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Warnings
- For external use only.
Directions
- To decrease bacteria on skin, apply small amount, covering hands with product for 30 seconds, Add water, lather, rinse.
| FIRST MARK
FOAM ANTIMICROBIAL HAND CLEANER
triclosan liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Performance Food Group (127038714) |
Revised: 12/2021
Document Id: ee1295fb-c01b-4eae-b4b0-5b1a57d79acb
Set id: 66899ee8-4024-4e8a-b0f5-993ed0f7e56d
Version: 3
Effective Time: 20211210
Performance Food Group