HEALTHY ACCENTS GAS RELIEF- simethicone emulsion
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DZA Brands, LLC Gas Relief Drug Facts
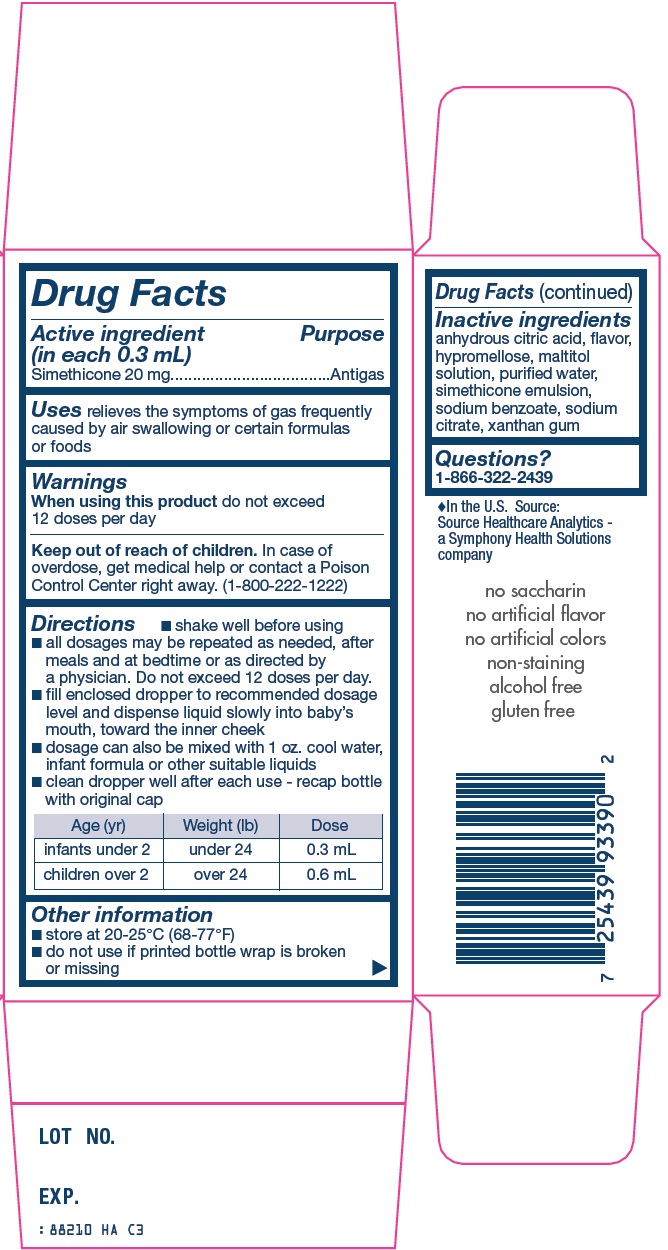
Directions
- •
- shake well before using
- •
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- •
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby’s mouth, toward the inner cheek
- •
- dosage can also be mixed with 1 oz. cool water, infant formula or other suitable liquids
- •
- clean dropper well after each use – recap bottle with original cap
|
Age (yr) |
Weight (lb) |
Dose |
|
infants under 2 |
under 24 |
0.3 mL |
|
children over 2 |
over 24 |
0.6 mL |
Other information
- •
- store at 20-25°C (68-77°F)
- •
- do not use if printed bottle wrap is broken or missing
Inactive ingredients
anhydrous citric acid, flavor, hypromellose, maltitol solution, purified water, simethicone emulsion, sodium benzoate, sodium citrate, xanthan gum
Principal Display Panel
infants’
gas relief
simethicone 20mg
anti-gas drops
gently breaks down baby’s gas bubbles
safe enough to use at every feeding*
*use as directed
Pediatrician Recommended #1 Active Ingredient
Compare to Infants’ Mylicon® Drops active ingredient
1 FL OZ (30mL)
100 infant doses – dropper enclosed
dye free drops – non-staining
| HEALTHY ACCENTS GAS RELIEF
simethicone emulsion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - DZA Brands LLC (090322194) |