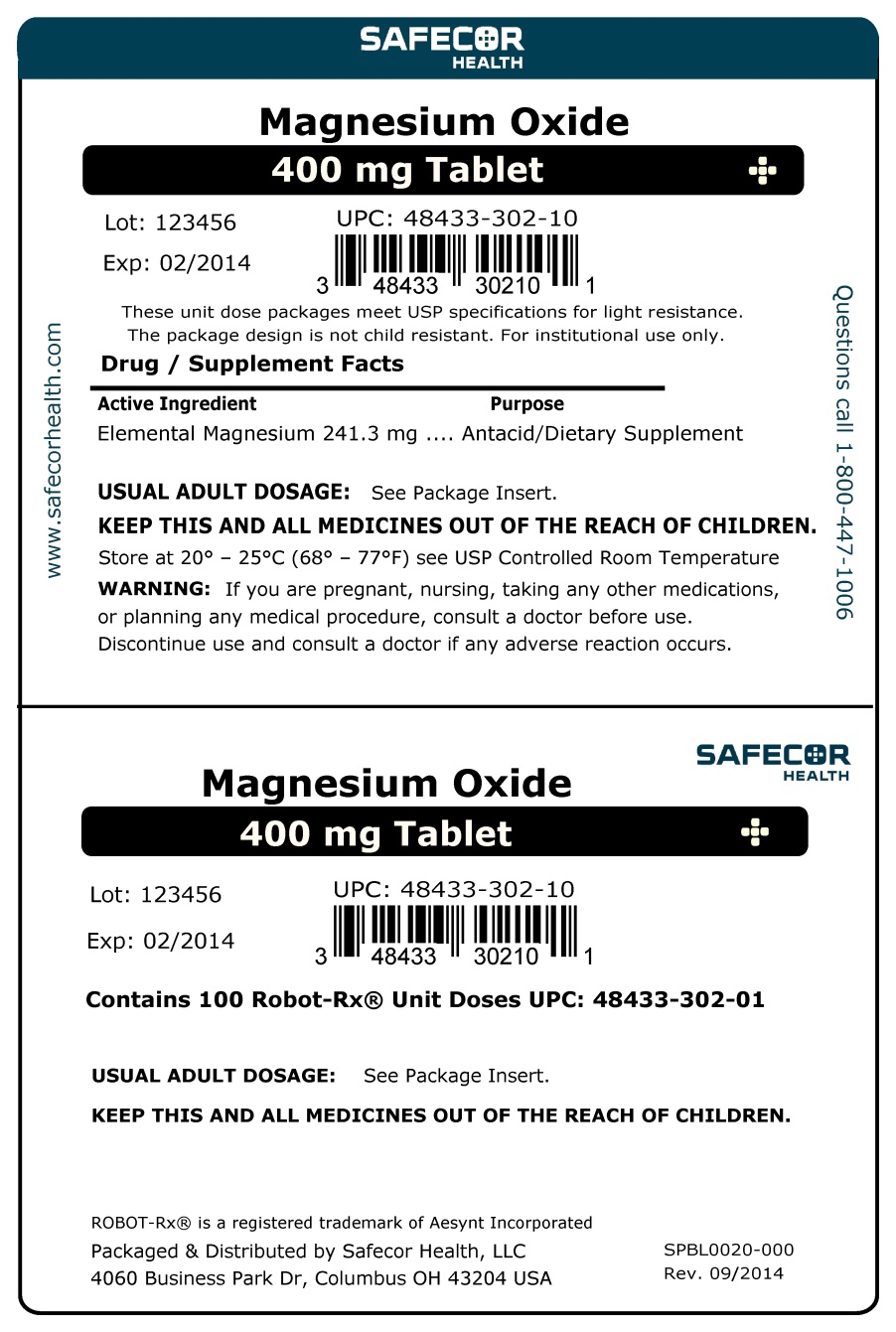
MAGNESIUM OXIDE- magnesium oxide tablet
Safecor Health, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Magnesium Oxide Tablets
WARNINGS
Do Not Use
Do not take more than 2 tablets in a 24 hour period or use the maximum dosage of this product for more than two weeks, except under the advice and supervision of a physician. May have a laxative effect.
DIRECTIONS
Antacid Directions: take 1 tablet twice a day or as directed by a physician
Magnesium Supplement Directions: take 1 to 2 tablets daily or as directed by a physician
OTHER INFORMATION
store at room temperature 59°-86° F (15°-30°C) , Magnesium content per tablet: 240 mg
| MAGNESIUM OXIDE
magnesium oxide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Safecor Health, LLC (828269675) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Safecor Health, LLC | 828269675 | REPACK(48433-302) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Safecor Health, LLC | 078805287 | REPACK(48433-302) | |
Revised: 11/2019
Document Id: 283657c8-ccba-49aa-b6e8-aed017315c07
Set id: 640680ef-b856-467f-bbaf-1144b290d75d
Version: 2
Effective Time: 20191123
Safecor Health, LLC