Label: CLINDAMYCIN HYDROCHLORIDE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 67296-0315-3 - Packager: RedPharm Drug Inc.
- This is a repackaged label.
- Source NDC Code(s): 63304-692
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 24, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Because clindamycin therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate, as described in the INDICATIONS AND USAGE section. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections. Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of therapy with clindamycin. -
DESCRIPTION
Clindamycin hydrochloride is the hydrated hydrochloride salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compound lincomycin.
The chemical name for clindamycin hydrochloride is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside monohydrochloride. The structural formula is represented below: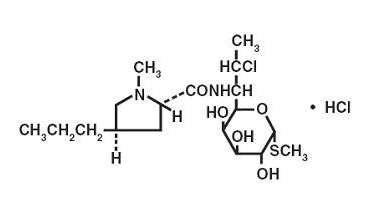
C18H33ClN2O5S•HCl M.W. 461.45
Clindamycin hydrochloride capsules, USP contain clindamycin hydrochloride equivalent to 150 mg or 300 mg of clindamycin. Each capsule also contains the following inactive ingredients: lactose monohydrate, corn starch, talc, and magnesium stearate. The empty hard gelatin capsule shell consists of FD and C Blue No. 1, titanium dioxide, gelatin and sodium lauryl sulphate. In addition 150 mg also contains yellow iron oxide. The capsules are printed with edible ink containing black iron oxide and shellac.
-
CLINICAL PHARMACOLOGY
Human Pharmacology
Serum level studies with a 150 mg oral dose of clindamycin hydrochloride in 24 normal adult volunteers showed that clindamycin was rapidly absorbed after oral administration. An average peak serum level of 2.5 mcg/mL was reached in 45 minutes; serum levels averaged 1.51 mcg/mL at 3 hours and 0.7 mcg/mL at 6 hours. Absorption of an oral dose is virtually complete (90%), and the concomitant administration of food does not appreciably modify the serum concentrations; serum levels have been uniform and predictable from person to person and dose to dose. Serum level studies following multiple doses of clindamycin hydrochloride for up to 14 days show no evidence of accumulation or altered metabolism of drug.
Serum half-life of clindamycin is increased slightly in patients with markedly reduced renal function. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Concentrations of clindamycin in the serum increased linearly with increased dose. Serum levels exceed the MIC (minimum inhibitory concentration) for most indicated organisms for at least six hours following administration of the usually recommended doses. Clindamycin is widely distributed in body fluids and tissues (including bones). The average biological half-life is 2.4 hours. Approximately 10% of the bioactivity is excreted in the urine and 3.6% in the feces; the remainder is excreted as bioinactive metabolites.
Doses of up to 2 grams of clindamycin per day for 14 days have been well tolerated by healthy volunteers, except that the incidence of gastrointestinal side effects is greater with the higher doses.
No significant levels of clindamycin are attained in the cerebrospinal fluid, even in the presence of inflamed meninges.
Pharmacokinetic studies in elderly volunteers (61 to 79 years) and younger adults (18 to 39 years) indicate that age alone does not alter clindamycin pharmacokinetics (clearance, elimination half-life, volume of distribution, and area under the serum concentration-time curve) after IV administration of clindamycin phosphate. After oral administration of clindamycin hydrochloride, elimination half-life is increased to approximately 4 hours (range 3.4 to 5.1 h) in the elderly compared to 3.2 hours (range 2.1 to 4.2 h) in younger adults. The extent of absorption, however, is not different between age groups and no dosage alteration is necessary for the elderly with normal hepatic function and normal (age-adjusted) renal function.Microbiology
Clindamycin inhibits bacterial protein synthesis by binding to the 50S subunit of the ribosome. It has activity against Gram-positive aerobes and anaerobes as well as the Gram-negative anaerobes. Clindamycin is bacteriostatic. Cross-resistance between clindamycin and lincomycin is complete. Antagonism in vitro has been demonstrated between clindamycin and erythromycin.
Clindamycin has been shown to be active against most of the isolates of the following microorganisms, both in vitro and in clinical infections, as described in the INDICATIONS AND USAGE section.
Gram-positive aerobes
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenes
Anaerobes
Prevotella melaninogenica
Fusobacterium necrophorum
Fusobacterium nucleatum
Peptostreptococcus anaerobius
Clostridium perfringens
The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for clindamycin. However, the safety and effectiveness of clindamycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Gram-positive aerobes
Staphylococcus epidermidis (methicillin-susceptible strains)
Streptococcus agalactiae
Streptococcus anginosus
Streptococcus oralis
Streptococcus mitis
Anaerobes
Prevotella intermedia
Prevotella bivia
Propionibacterium acnes
Micromonas (“Peptostreptococcus”) micros
Finegoldia (“Peptostreptococcus”) magna
Actinomyces israelii
Clostridium clostridioforme
Eubacterium lentum
Susceptibility Testing Methods
NOTE: Susceptibility testing by dilution methods requires the use of clindamycin susceptibility powder.
When available, the results of in vitro susceptibility tests should be provided to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth and agar)1,2,3 or equivalent with standardized inoculum concentrations and standardized concentrations of clindamycin powder. The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion Techniques
Quantitative methods that require the measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2,3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 2 mcg of clindamycin to test the susceptibility of microorganisms to clindamycin. The disk diffusion interpretive criteria are provided in Table 1.
Table 1. Susceptibility Interpretive Criteria for Clindamycin Pathogen Susceptibility Interpretive Criteria Minimal Inhibitory
Concentrations
(MIC in mcg/mL)Disk Diffusion
(Zone Diameters in mm)
S I R S I R Staphylococcus spp.
≤0.5
1-2
≥4
≥21
15-20
≤14
Streptococcus pneumoniae and
other Streptococcus spp.
≤0.25a
0.5
≥1
≥19b
16-18
≤15
Anaerobic Bacteriac
≤2
4
≥8
NA
NA
NA
a These interpretive standards for S. pneumoniae and other Streptococcus spp. are applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35°C for 20 to 24 hours.
b These zone diameter interpretive standards are applicable only to tests performed using Mueller-Hinton agar supplemented with 5% sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35°C for 20 to 24 hours.
c These interpretive criteria are for all anaerobic bacterial pathogens; no organism specific interpretive criteria are available.
NA=Not applicable
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of quality control microorganisms to control the technical aspects of the test procedures. Standard clindamycin powder should provide the following range of values noted in Table 2. NOTE: Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within bacteria; the specific strains used for microbiological quality control are not clinically significant.
Table 2. Acceptable Quality Control Ranges for Clindamycin to be Used in Validation of Susceptibility Test Results
QC StrainAcceptable Quality Control Ranges Minimum Inhibitory
Concentrations
(MIC in mcg/mL)Disk Diffusion
(Zone Diameters in mm)When Testing Aerobic Pathogens
Staphylococcus aureus ATCC 29213
0.06–0.25
NA
Staphylococcus aureus ATCC 25923
NA
24–30
Streptococcus pneumoniae ATCC 49619d
0.03–0.12e
19–25f
When Testing Strict Anaerobes
Bacteroides fragilis ATCC 25285
0.5–2
NA
Bacteroides thetaiotaomicron ATCC 29741
2–8
NA
Eubacterium lentum ATCC 43055
0.06–0.25
NA
NA=Not applicable
d This organism may be used for validation of susceptibility test results when testing Streptococcus spp. other than S. pneumoniae.
e This quality control range for S. pneumoniae is applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35°C for 20 to 24 hours.
f This quality control zone diameter range is applicable only to tests performed using Mueller-Hinton agar supplemented with 5% sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35°C for 20 to 24 hours.
ATCC® is a registered trademark of the American Type Culture CollectionINDICATIONS AND USAGE
Clindamycin hydrochloride capsules are indicated in the treatment of serious infections caused by susceptible anaerobic
-
INDICATIONS AND USAGE
Clindamycin hydrochloride capsules are indicated in the treatment of serious infections caused by susceptible anaerobic bacteria.
Clindamycin hydrochloride capsules are also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, as described in the WARNING box, before selecting clindamycin the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, nongonococcal tuboovarian abscess, pelvic cellulitis and postsurgical vaginal cuff infection.
Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Pneumococci: Serious respiratory tract infections.
Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
See WARNING box.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
Usage in Meningitis—Since clindamycin does not diffuse adequately into the cerebrospinal fluid, the drug should not be used in the treatment of meningitis. -
PRECAUTIONS
General
Review of experience to date suggests that a subgroup of older patients with associated severe illness may tolerate diarrhea less well. When clindamycin is indicated in these patients, they should be carefully monitored for change in bowel frequency.
Clindamycin hydrochloride should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Clindamycin hydrochloride should be prescribed with caution in atopic individuals.
Indicated surgical procedures should be performed in conjunction with antibiotic therapy.
The use of clindamycin hydrochloride occasionally results in overgrowth of nonsusceptible organisms—particularly yeasts. Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation.
Clindamycin dosage modification may not be necessary in patients with renal disease. In patients with moderate to severe liver disease, prolongation of clindamycin half-life has been found. However, it was postulated from studies that when given every eight hours, accumulation should rarely occur. Therefore, dosage modification in patients with liver disease may not be necessary. However, periodic liver enzyme determinations should be made when treating patients with severe liver disease.
Prescribing clindamycin hydrochloride in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
Information for Patients
Patients should be counseled that antibacterial drugs including clindamycin hydrochloride should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clindamycin hydrochloride is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clindamycin hydrochloride or other antibacterial drugs in the future.
- Laboratory Tests
-
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Antagonism has been demonstrated between clindamycin and erythromycin in vitro. Because of possible clinical significance, these two drugs should not be administered concurrently.
-
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Fertility studies in rats treated orally with up to 300 mg/kg/day (approximately 1.6 times the highest recommended adult human dose based on mg/m2) revealed no effects on fertility or mating ability.
-
Pregnancy
Teratogenic effects
Pregnancy Category B
Reproduction studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (3.2 and 1.6 times the highest recommended adult human dose based on mg/m2, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (1.3 and 0.7 times the highest recommended adult human dose based on mg/m2, respectively) revealed no evidence of teratogenicity.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed. - Nursing Mothers
- Pediatric Use
-
Geriatric Use
Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients. However, other reported clinical experience indicates that antibiotic-associated colitis and diarrhea (due to Clostridium difficile) seen in association with most antibiotics occur more frequently in the elderly (>60 years) and may be more severe. These patients should be carefully monitored for the development of diarrhea.
Pharmacokinetic studies with clindamycin have shown no clinically important differences between young and elderly subjects with normal hepatic function and normal (age-adjusted) renal function after oral or intravenous administration.
-
ADVERSE REACTIONS
The following reactions have been reported with the use of clindamycin.
Gastrointestinal: Abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting and diarrhea (see WARNING box). The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS).
Hypersensitivity Reactions: Generalized mild to moderate morbilliform-like (maculopapular) skin rashes are the most frequently reported adverse reactions. Vesiculobullous rashes, as well as urticaria, have been observed during drug therapy. Rare instances of erythema multiforme, some resembling Stevens-Johnson syndrome, and a few cases of anaphylactoid reactions have also been reported.
Skin and Mucous Membranes: Pruritus, vaginitis, and rare instances of exfoliative dermatitis have been reported. (See Hypersensitivity Reactions.)
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Renal: Although no direct relationship of clindamycin to renal damage has been established, renal dysfunction as evidenced by azotemia, oliguria, and/or proteinuria has been observed in rare instances.
Hematopoietic: Transient neutropenia (leukopenia) and eosinophilia have been reported. Reports of agranulocytosis and thrombocytopenia have been made. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of the foregoing.
Musculoskeletal: Rare instances of polyarthritis have been reported.
-
OVERDOSAGE
Significant mortality was observed in mice at an intravenous dose of 855 mg/kg and in rats at an oral or subcutaneous dose of approximately 2618 mg/kg. In the mice, convulsions and depression were observed.
Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
-
DOSAGE AND ADMINISTRATION
If significant diarrhea occurs during therapy, this antibiotic should be discontinued (see WARNING box).
Adults
Serious infections—150 to 300 mg every 6 hours. More severe infections—300 to 450 mg every 6 hours. Pediatric Patients
Serious infections—8 to 16 mg/kg/day (4 to 8 mg/lb/day) divided into three or four equal doses. More severe infections—16 to 20 mg/kg/day (8 to 10 mg/lb/day) divided into three or four equal doses.
To avoid the possibility of esophageal irritation, clindamycin hydrochloride capsules should be taken with a full glass of water.
Serious infections due to anaerobic bacteria are usually treated with clindamycin phosphate injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin hydrochloride capsules.
In cases of β-hemolytic streptococcal infections, treatment should continue for at least 10 days.
-
HOW SUPPLIED
Clindamycin hydrochloride capsules are available in the following strengths, colors and sizes:
150 mg Turquoise blue opaque cap and light green body printed with “RX692” on cap and body in black ink.
NDC 67296-0315-3 Bottles of 40
Store at 20 - 25° C (68 - 77° F). (See USP Controlled Room Temperature).
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
One year oral toxicity studies in Spartan Sprague-Dawley rats and beagle dogs at dose levels up to 300 mg/kg/day (approximately 1.6 and 5.4 times the highest recommended adult human dose based on mg/m2, respectively) have shown clindamycin to be well tolerated. No appreciable difference in pathological findings has been observed between groups of animals treated with clindamycin and comparable control groups. Rats receiving clindamycin hydrochloride at 600 mg/kg/day (approximately 3.2 times the highest recommended adult human dose based on mg/m2) for 6 months tolerated the drug well; however, dogs dosed at this level (approximately 10.8 times the highest recommended adult human dose based on mg/m2) vomited, would not eat, and lost weight.
-
REFERENCES
- NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-5th ed. NCCLS document M7-A5, 2000. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898.
- NCCLS. Performance Standards for Antimicrobial Susceptibility Testing: 13th Informational Supplement. NCCLS document M100-S13 (M2 and M7), 2003. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898.
- NCCLS. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria 5th ed. Approved Standard. NCCLS document M11-A5, 2001. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898.
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-8th ed. NCCLS document M2-A8 (ISBN 1-56238-393-0), 2003. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898.
Manufactured for:
Ranbaxy Pharmaceuticals Inc.
Jacksonville, FL 32257 USA
by: Ohm Laboratories Inc.
North Brunswick, NJ 08902 USA
November 2006
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN HYDROCHLORIDE
clindamycin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67296-0315(NDC:63304-692) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN HYDROCHLORIDE (UNII: T20OQ1YN1W) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN HYDROCHLORIDE 150 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue (Turquoise blue) , green (light green) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code RX692 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67296-0315-3 40 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065442 11/01/2006 Labeler - RedPharm Drug Inc. (008039641) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 051565745 manufacture


