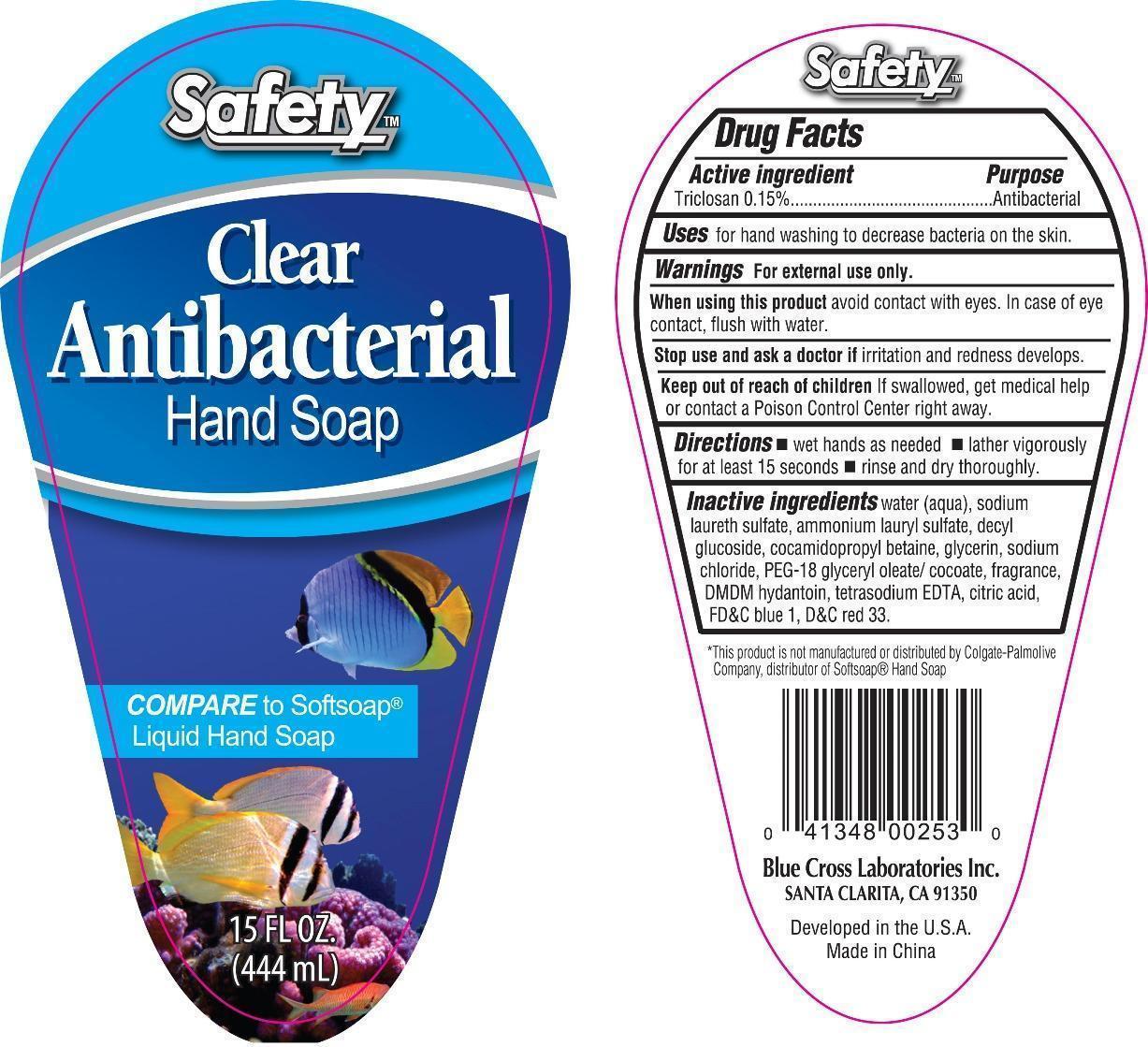
CLEAR ANTIBACTERIAL HAND- triclosan soap
Blue Cross Laboratories, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clear Antibacterial Hand Soap
Active ingredient Purpose
Triclosan 0.15% Antibacterial
Uses for hand washing to decrease to decrease bacteria on the skin
| CLEAR ANTIBACTERIAL HAND
triclosan soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Blue Cross Laboratories, Inc (008298879) |
| Registrant - Blue Cross Laboratories, Inc (008298879) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Liyuan Daily Chemical Products Co., Ltd. | 530766098 | manufacture(22431-582) | |
Revised: 1/2021
Document Id: b82b6db1-f4d3-c296-e053-2a95a90a9093
Set id: 63241c2f-55f0-4c33-8917-c9de024ba352
Version: 5
Effective Time: 20210105
Blue Cross Laboratories, Inc