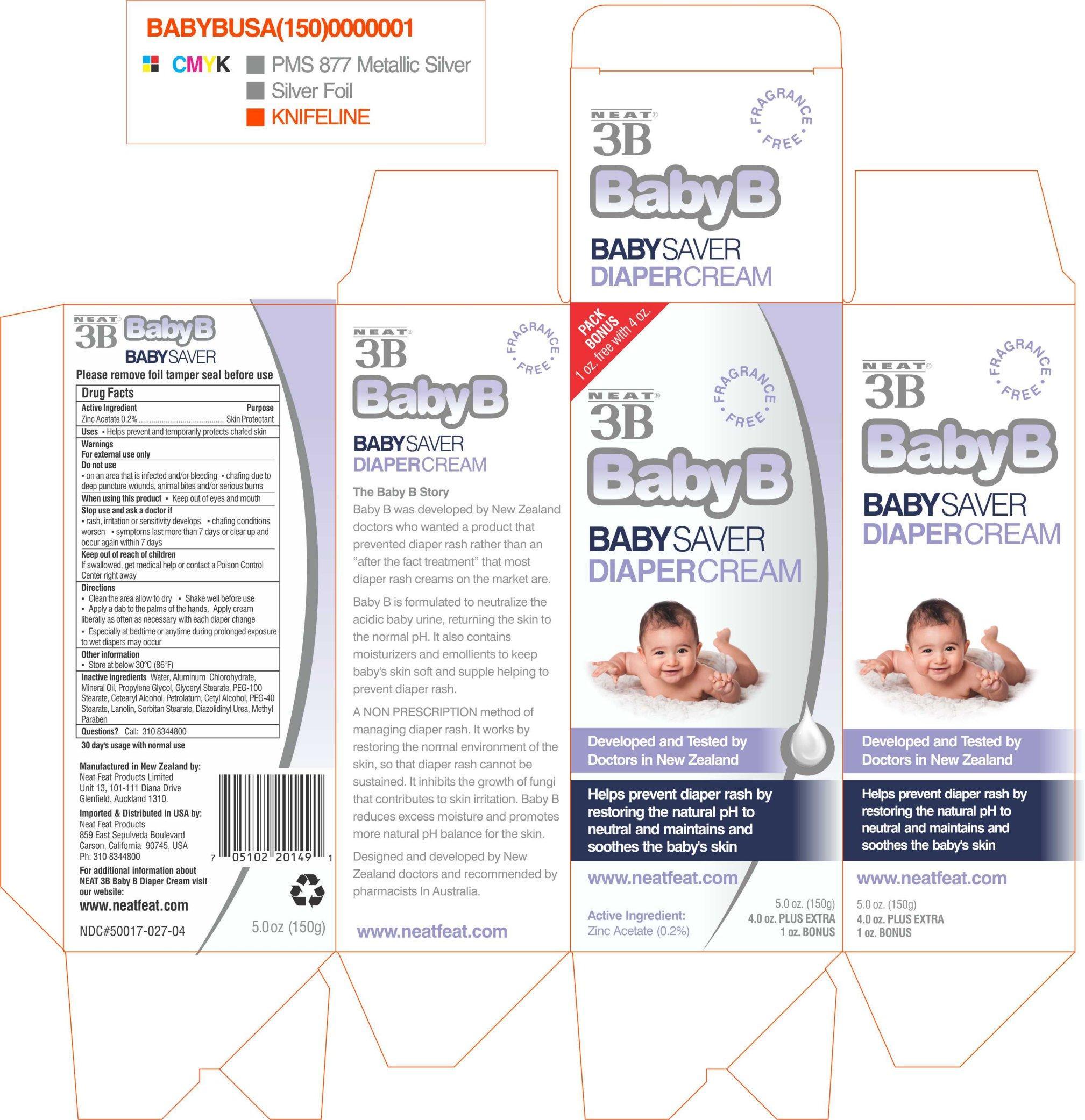
NEAT 3B BABY B- skin protectant cream
Neat Feat Products Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NEAT® 3B BABY B DIAPER
Keep out of reach of children
if swallowed, get medical help or contact a Poison Control Center right away.
Warnings
For external use only
Do not use
on an area that is infected and/or bleeding
chafing due to deep puncture wounds, animal bites and/or serious burns
When using this product
Keep out of eyes and mouth
Stop use and ask a doctor if
Rash, irritation or sensitivity develops
chafing conditions worsen
symptoms last more than 7 days or clear up and occur again withing 7 days
Directions
Clean area and allow to dry
Shake well before use
Apply a dab to palms of the hands
Apply cream liberally as often as necessary with each diaper change
Especially at bedtime or anytime during prolonged exposure to wet diapers may occur
| NEAT 3B BABY B
skin protectant cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Neat Feat Products Limited (590412409) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tripak Pharmaceuticals | 758658819 | manufacture(50017-027) , pack(50017-027) , label(50017-027) | |