METHYLPHENIDATE HYDROCHLORIDE- methylphenidate hydrochloride tablet
CorePharma, LLC
----------
Methylphenidate Hydrochloride Tablets, USP
5 mg, 10 mg and 20 mg
CII
Rx only
DESCRIPTION
Methylphenidate hydrochloride, USP is a mild central nervous system (CNS) stimulant, available as tablets of 5, 10, and 20 mg for oral administration. Methylphenidate hydrochloride is methyl α-phenyl-2-piperidineacetate hydrochloride, and its structural formula is:
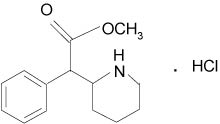
Methylphenidate hydrochloride, USP is a white to off white powder. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77.
Inactive Ingredients: Lactose monohydrate, Microcrystalline Cellulose, Stearic Acid and FD&C Yellow No. 6 Aluminum Lake.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Methylphenidate hydrochloride is a mild central nervous system stimulant.
The mode of action in man is not completely understood, but methylphenidate hydrochloride presumably activates the brain stem arousal system and cortex to produce its stimulant effect.
There is neither specific evidence which clearly establishes the mechanism whereby methylphenidate hydrochloride produces its mental and behavioral effects in children, nor conclusive evidence regarding how these effects relate to the condition of the central nervous system.
Effects on QT Interval
The effect of Focalin® XR (dexmethylphenidate, the pharmacologically active d-enantiomer of methylphenidate hydrochloride) on the QT interval was evaluated in a double-blind, placebo- and open label active (moxifloxacin)-controlled study following single doses of Focalin® XR 40 mg in 75 healthy volunteers. ECGs were collected up to 12 hours postdose. Frederica’s method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was <5 ms, and the upper limit of the 90% confidence interval was below 10 ms for all time matched comparisons versus placebo. This was below the threshold of clinical concern and there was no evident-exposure response relationship.
INDICATIONS
Attention Deficit Disorders, Narcolepsy
Attention Deficit Disorders (previously known as Minimal Brain Dysfunction in Children). Other terms being used to describe the behavioral syndrome below include: Hyperkinetic Child Syndrome, Minimal Brain Damage, Minimal Cerebral Dysfunction, Minor Cerebral Dysfunction.
Methylphenidate hydrochloride tablets, USP are indicated as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate-to-severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis of this syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted.
Special Diagnostic Considerations
Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources.
Characteristics commonly reported include: chronic history of short attention span, distractibility, emotional lability, impulsivity, and moderate-to-severe hyperactivity; minor neurological signs and abnormal EEG. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of one or more of these characteristics.
Drug treatment is not indicated for all children with this syndrome. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is generally necessary. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the child’s symptoms.
CONTRAINDICATIONS
Marked anxiety, tension, and agitation are contraindications to methylphenidate hydrochloride, since the drug may aggravate these symptoms. Methylphenidate hydrochloride is contraindicated also in patients known to be hypersensitive to the drug, in patients with glaucoma, and in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.
Methylphenidate hydrochloride is contraindicated during treatment with monoamine oxidase inhibitors, and also within a minimum of 14 days following discontinuation of a monoamine oxidase inhibitor (hypertensive crises may result).
WARNINGS
Serious Cardiovascular Events
Sudden Death and Preexisting Structural Cardiac Abnormalities or Other Serious Heart Problems
Children and Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Adults
Sudden death, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs.
Hypertension and Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2 to 4 mmHg) and average heart rate (about 3 to 6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with preexisting hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia.
Assessing Cardiovascular Status in Patients being Treated with Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Preexisting Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/ manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3,482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
Long-Term Suppression of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or during discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
Peripheral Vasculopathy, Including Raynaud's Phenomenon
Stimulants, including methylphenidate hydrochloride tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
Use in Children Under Six Years of Age
Methylphenidate hydrochloride should not be used in children under 6 years, since safety and efficacy in this age group have not been established.
Drug Dependence
Methylphenidate hydrochloride should be given cautiously to patients with a history of drug dependence or alcoholism. Chronic abusive use can lead to marked tolerance and psychological dependence with varying degrees of abnormal behavior. Frank psychotic episodes can occur, especially with parenteral abuse. Careful supervision is required during withdrawal from abusive use, since severe depression may occur. Withdrawal following chronic therapeutic use may unmask symptoms of the underlying disorder that may require follow-up.
PRECAUTIONS
Patients with an element of agitation may react adversely; discontinue therapy if necessary.
Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
Drug treatment is not indicated in all cases of this behavioral syndrome and should be considered only in light of the complete history and evaluation of the child. The decision to prescribe methylphenidate hydrochloride should depend on the physician’s assessment of the chronicity and severity of the child’s symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with methylphenidate hydrochloride is usually not indicated.
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with methylphenidate and should counsel them in its appropriate use. A patient Medication Guide is available for methylphenidate hydrochloride. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Priapism
- Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism.
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]
- Instruct patients beginning treatment with methylphenidate hydrochloride tablets about the risk of peripheral vasculopathy, including Raynaud’s Phenomenon, and in associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride tablets.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Drug Interactions
Methylphenidate hydrochloride should not be used in patients being treated (currently or within the proceeding two weeks) with MAO Inhibitors (see CONTRAINDICATIONS, Monoamine Oxidase Inhibitors). Because of possible effects on blood pressure, methylphenidate hydrochloride should be used cautiously with pressor agents.
Methylphenidate may decrease the effectiveness of drugs used to treat hypertension. Methylphenidate is metabolized primarily to ritalinic acid by de-esterification and not through oxidative pathways.
Human pharmacologic studies have shown that racemic methylphenidate may inhibit the metabolism of coumarin anticoagulants, anticonvulsants (e.g., phenobarbital, phenytoin, primidone), and tricyclic drugs (e.g., imipramine, clomipramine, desipramine). Downward dose adjustments of these drugs may be required when given concomitantly with methylphenidate. It may be necessary to adjust the dosage and monitor plasma drug concentration (or, in case of coumarin, coagulation times), when initiating or discontinuing methylphenidate.
Carcinogenesis/Mutagenesis/Impairment of Fertility
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas, at a daily dose of approximately 60 mg/kg/day. This dose is approximately 30 times and 4 times the maximum recommended human dose on a mg/kg and mg/m2 basis, respectively. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Methylphenidate did not cause any increases in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 22 times and 5 times the maximum recommended human dose on a mg/kg and mg/m2 basis, respectively.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or in the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary (CHO) cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 80-fold and 8-fold the highest recommended dose on a mg/kg and mg/m2 basis, respectively.
PREGNANCY
Pregnancy Category C
In studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Teratogenic effects (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 40 times the maximum recommended human dose (MRHD) on a mg/m2 basis. The no effect level for embryofetal development in rabbits was 60 mg/kg/day (11 times the MRHD on a mg/m2 basis). There was no evidence of specific teratogenic activity in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (7 times the MRHD on a mg/m2 basis), which was also maternally toxic. The no effect level for embryofetal development in rats was 25 mg/kg/day (2 times the MRHD on a mg/m2 basis). When methylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 45 mg/kg/day, offspring body weight gain was decreased at the highest dose (4 times the MRHD on a mg/m2 basis), but no other effects on postnatal development were observed. The no effect level for pre- and postnatal development in rats was 15 mg/kg/day (equal to the MRHD on a mg/m2 basis).
Adequate and well-controlled studies in pregnant women have not been conducted. Methylphenidate hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether methylphenidate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if methylphenidate hydrochloride is administered to a nursing woman.
Pediatric Use
Methylphenidate hydrochloride should not be used in children under six years of age (see WARNINGS).
In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (Postnatal Day 7) and continuing through sexual maturity (Postnatal Week 10). When these animals were tested as adults (Postnatal Weeks 13-14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 6 times the maximum recommended human dose [MRHD] on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (12 times the MRHD on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (half the MRHD on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
ADVERSE REACTIONS
Nervousness and insomnia are the most common adverse reactions but are usually controlled by reducing dosage and omitting the drug in the afternoon or evening. Other reactions include hypersensitivity (including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathological findings of necrotizing vasculitis, and thrombocytopenic purpura); anorexia; nausea; dizziness; palpitations; headache; dyskinesia; drowsiness; blood pressure and pulse changes, both up and down; tachycardia; angina; cardiac arrhythmia; abdominal pain; weight loss during prolonged therapy; libido changes. There have been rare reports of Tourette’s syndrome. Toxic psychosis has been reported. Although a definite causal relationship has not been established, the following have been reported in patients taking this drug: serotonin syndrome in combination with serotonergic drugs, rhabdomyolysis, instances of abnormal liver function, ranging from transaminase elevation to severe hepatic injury; isolated cases of cerebral arteritis and/or occlusion; leukopenia and/or anemia; transient depressed mood; aggressive behavior; a few instances of scalp hair loss. Very rare reports of neuroleptic malignant syndrome (NMS) have been received, and, in most of these, patients were concurrently receiving therapies associated with NMS. In a single report, a 10-year-old boy who had been taking methylphenidate for approximately 18 months experienced an NMS-like event within 45 minutes of ingesting his first dose of venlafaxine. It is uncertain whether this case represented a drug-drug interaction, a response to either drug alone, or some other cause.
In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia, and tachycardia may occur more frequently; however, any of the other adverse reactions listed above may also occur.
DOSAGE AND ADMINISTRATION
Dosage should be individualized according to the needs and responses of the patient.
Adults
Tablets: Administer in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Average dosage is 20 to 30 mg daily. Some patients may require 40 to 60 mg daily. In others, 10 to 15 mg daily will be adequate. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m.
Children (6 years and over)
Methylphenidate hydrochloride tablets should be initiated in small doses, with gradual weekly increments. Daily dosage above 60 mg is not recommended.
If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
Tablets: Start with 5 mg twice daily (before breakfast and lunch) with gradual increments of 5 to 10 mg weekly.
If paradoxical aggravation of symptoms or other adverse effects occur, reduce dosage, or, if necessary, discontinue the drug.
Methylphenidate hydrochloride tablets should be periodically discontinued to assess the child’s condition. Improvement may be sustained when the drug is either temporarily or permanently discontinued.
Drug treatment should not and need not be indefinite and usually may be discontinued after puberty.
OVERDOSAGE
Signs and symptoms of acute overdosage, resulting principally from overstimulation of the central nervous system and from excessive sympathomimetic effects, may include the following: vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes. Rhabdomyolysis has also been reported in overdose.
Consult with a Certified Poison Control Center regarding treatment for up-to-date guidance and advice.
Treatment consists of appropriate supportive measures. The patient must be protected against self-injury and against external stimuli that would aggravate overstimulation already present. Gastric contents may be evacuated by gastric lavage. In the presence of severe intoxication, use a carefully titrated dosage of a short-acting barbiturate before performing gastric lavage. Other measures to detoxify the gut include administration of activated charcoal and a cathartic.
Intensive care must be provided to maintain adequate circulation and respiratory exchange; external cooling procedures may be required for hyperpyrexia.
Efficacy of peritoneal dialysis or extracorporeal hemodialysis for methylphenidate hydrochloride overdosage has not been established.
HOW SUPPLIED
Methylphenidate hydrochloride tablets USP, 5 mg are Light Orange to Orange, Round Compressed Tablets. Debossed cor on one side and 237 on the other side.
They are supplied as follows:
Bottles of 100.........................................NDC 64720-237-10
Methylphenidate hydrochloride tablets USP, 10 mg are Light Orange to Orange, Round Compressed Tablets. Debossed cor over 238 on one side and bisect on the other side.
They are supplied as follows:
Bottles of 100........................................ NDC 64720-238-10
Methylphenidate hydrochloride tablets USP, 20 mg are Light Orange to Orange, Round Compressed Tablets. Debossed cor over 239 on one side and bisect on the other side.
They are supplied as follows:
Bottles of 100.........................................NDC 64720-239-10
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light.
Dispense in tight, light-resistant container as defined in the USP.
LB # 691-05
Rev. January, 2017

Dist. by:
CorePharma, LLC
Middlesex, NJ 08846
For additional copies of the printed patient information/medication guide, please visit www.corepharma.com or call 1-800-850-2719.
MEDICATION GUIDE
Methylphenidate Hydrochloride Tablets, USP CII
(meth" il fen' i date hye" droe klor' ide)
Read the Medication Guide that comes with methylphenidate hydrochloride tablets before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your or your child’s treatment with methylphenidate hydrochloride tablets.
What is the most important information I should know about methylphenidate hydrochloride tablets?
The following have been reported with use of methylphenidate hydrochloride and other stimulant medicines.
1. Heart-related problems:
• sudden death in patients who have heart problems or heart defects
• stroke and heart attack in adults
• increased blood pressure and heart rate
Tell your doctor if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your doctor should check you or your child carefully for heart problems before starting methylphenidate hydrochloride tablets.
Your doctor should check your or your child’s blood pressure and heart rate regularly during treatment with methylphenidate hydrochloride tablets.
Call your doctor right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking methylphenidate hydrochloride tablets.
2. Mental (Psychiatric) problems:
All Patients
• new or worse behavior and thought problems
• new or worse bipolar illness
• new or worse aggressive behavior or hostility
Children and Teenagers
• new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your doctor right away if you or your child have any new or worsening mental symptoms or problems while taking methylphenidate hydrochloride tablets, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red
• Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in the fingers or toes.
• Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride tablets.
What are methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets are a central nervous system stimulant prescription medicine. It is used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Methylphenidate hydrochloride tablets may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Methylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Methylphenidate hydrochloride tablets are also used in the treatment of a sleep disorder called narcolepsy.
Methylphenidate hydrochloride tablets are a federally controlled substance (CII) because it can be abused or lead to dependence. Keep methylphenidate hydrochloride tablets in a safe place to prevent misuse and abuse. Selling or giving away methylphenidate hydrochloride tablets may harm others, and is against the law.
Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets should not be taken if you or your child:
• are very anxious, tense, or agitated
• have an eye problem called glaucoma
• have tics or Tourette’s syndrome, or a family history of Tourette’s syndrome. Tics are hard to control repeated movements or sounds.
• are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor or MAOI.
• are allergic to anything in methylphenidate hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients.
Methylphenidate hydrochloride tablets should not be used in children less than 6 years old because it has not been studied in this age group.
Methylphenidate hydrochloride tablets may not be right for you or your child. Before starting methylphenidate hydrochloride tablets tell your or your child’s doctor about all health conditions (or a family history of) including:
• heart problems, heart defects, high blood pressure
• mental problems including psychosis, mania, bipolar illness, or depression
• tics or Tourette’s syndrome
• seizures or have had an abnormal brain wave test (EEG)
• circulation problems in fingers or toes
Tell your doctor if you or your child is pregnant, planning to become pregnant, or breastfeeding.
Can methylphenidate hydrochloride tablets be taken with other medicines?
Tell your doctor about all of the medicines that you or your child takes including prescription and nonprescription medicines, vitamins, and herbal supplements. Methylphenidate hydrochloride tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride tablets.
Your doctor will decide whether methylphenidate hydrochloride tablets can be taken with other medicines.
Especially tell your doctor if you or your child takes:
• anti-depression medicines including MAOIs
• seizure medicines
• blood thinner medicines
• blood pressure medicines
• cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking methylphenidate hydrochloride tablets without talking to your doctor first.
How should methylphenidate hydrochloride tablets be taken?
• Take methylphenidate hydrochloride tablets exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
• Methylphenidate hydrochloride tablets are usually taken 2 to 3 times a day.
• Take methylphenidate hydrochloride tablets 30 to 45 minutes before a meal.
• From time to time, your doctor may stop methylphenidate hydrochloride tablets treatment for a while to check ADHD symptoms.
• Your doctor may do regular checks of the blood, heart, and blood pressure while taking methylphenidate hydrochloride tablets. Children should have their height and weight checked often while taking methylphenidate hydrochloride tablets. Methylphenidate hydrochloride tablets treatment may be stopped if a problem is found during these check-ups.
• If you or your child takes too much methylphenidate hydrochloride tablets or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of methylphenidate hydrochloride tablets?
See “What is the most important information I should know about methylphenidate hydrochloride tablets?” for information on reported heart and mental problems.
Other serious side effects include:
• slowing of growth (height and weight) in children
• seizures, mainly in patients with a history of seizures
• eyesight changes or blurred vision
• painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develop priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a doctor immediately.
Common side effects include:
• headache
• stomach ache
• trouble sleeping
• nausea
• decreased appetite
• nervousness
Talk to your doctor if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store methylphenidate hydrochloride tablets?
• Store methylphenidate hydrochloride tablets in a safe place at room temperature, 20° to 25°C (68° to 77°F). Protect from light.
• Keep methylphenidate hydrochloride tablets and all medicines out of the reach of children.
General information about methylphenidate hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use methylphenidate hydrochloride tablets for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride tablets to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about methylphenidate hydrochloride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about methylphenidate hydrochloride tablets that was written for healthcare professionals. For more information about methylphenidate hydrochloride tablets call 1-800-850-2719.
What are the ingredients in methylphenidate hydrochloride tablets?
Active Ingredient: methylphenidate HCl, USP
Inactive Ingredients: Lactose monohydrate, Microcrystalline Cellulose, Stearic Acid and FD&C Yellow No. 6 Aluminum Lake.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
LB # 693-03
Rev. September, 2016

Dist. by:
CorePharma, LLC
Middlesex, NJ 08846
For additional copies of the printed patient information/medication guide, please visit www.corepharma.com or call 1-800-850-2719.
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - CorePharma, LLC (031192276) |


