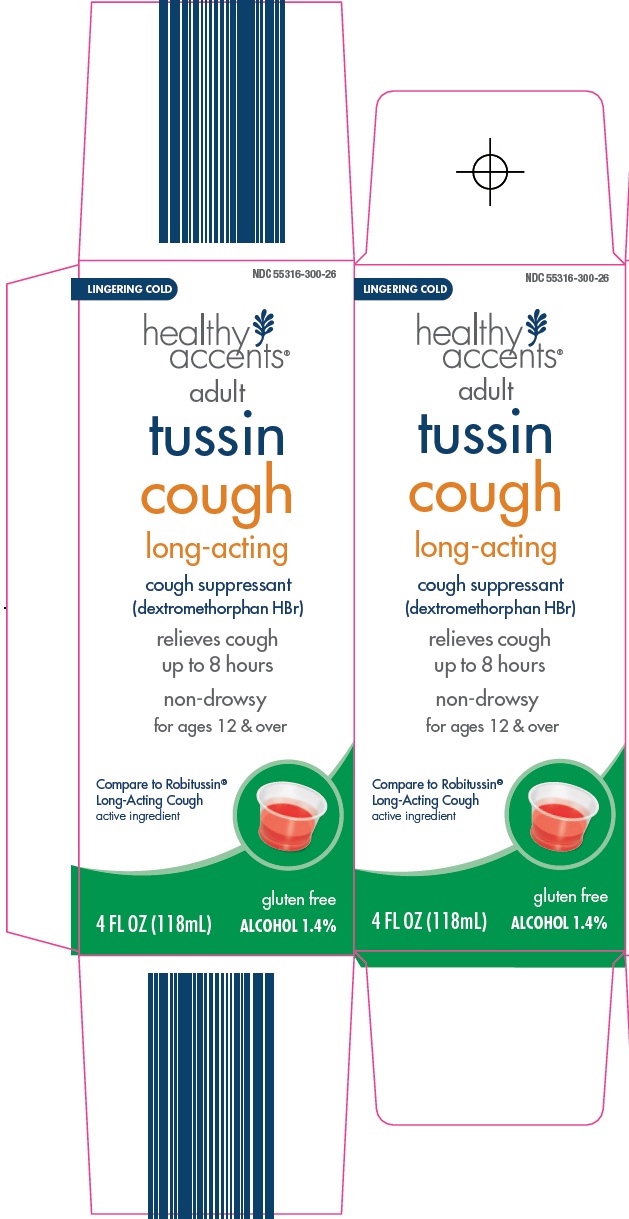
HEALTHY ACCENTS TUSSIN COUGH ADULT- dextromethorphan hydrobromide liquid
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DZA Brands, LLC Adult Tussin Cough Drug Facts
Uses
- •
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
Warnings
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Directions
- •
- do not take more than 4 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
10 mL every 6 to 8 hours |
|
children under 12 years |
do not use |
| HEALTHY ACCENTS TUSSIN COUGH
ADULT
dextromethorphan hydrobromide liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - DZA Brands LLC (090322194) |
Revised: 11/2019
Document Id: a940d7b1-292a-43b7-9d3f-cb36da6514dd
Set id: 5e5fa582-8a98-4fdc-899f-562b79a33cec
Version: 3
Effective Time: 20191115
DZA Brands LLC