ALLERGY EYE DROPS- naphazoline hydrochloride and pheniramine maleate solution/ drops
CVS Pharmacy
----------
Warnings
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- pupils may become enlarged temporarily
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- overuse may cause more eye redness
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to four times a day
- children under 6 years of age: consult a doctor
Other information
- some users may experience a brief tingling sensation
- store between 20° to 25°C (68° to 77°F)
Inactive ingredients
Boric Acid and Sodium Borate buffer system preserved with Benzalkonium Chloride (0.01%) and Edetate Disodium (0.1%), Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH) and Water for Injection.
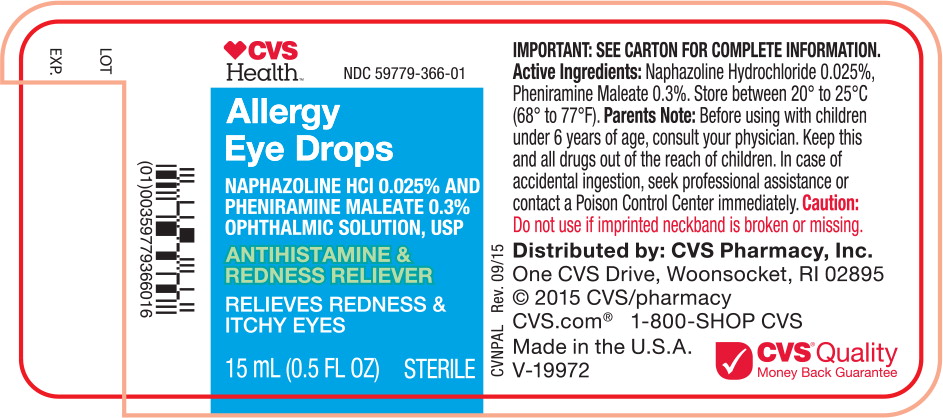
Principal Display Panel Text for Container Label:
CVS Health™ Logo NDC 59779-366-01
Allergy
Eye Drops
NAPHAZOLINE HCl 0.025% AND
PHENIRAMINE MALEATE 0.3%
OPHTHALMIC SOLUTION, USP
ANTIHISTAMINE &
REDNESS RELIEVER
RELIEVES REDNESS &
ITCHY EYES
15 mL (0.5 FL OZ) STERILE
Principal Display Panel Text for Carton Label:
CVS Health™ Logo Compare to the active
ingredient in Visine-A®*
NDC 59779-366-01
Allergy
Eye Drops
NAPHAZOLINE HCl 0.025% AND
PHENIRAMINE MALEATE 0.3%
OPHTHALMIC SOLUTION, USP
ANTIHISTAMINE & REDNESS
RELIEVER
RELIEVES REDNESS &
ITCHY EYES DUE TO:
• Pollen
• Ragweed
• Grass
• Animal hair
& dander
STERILE
15 mL (0.5 FL OZ)
| ALLERGY EYE DROPS
naphazoline hydrochloride and pheniramine maleate solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Akorn, Inc. (062649876) |