XYLISHIELD- sodium fluoride paste, dentifrice
Ultradent Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
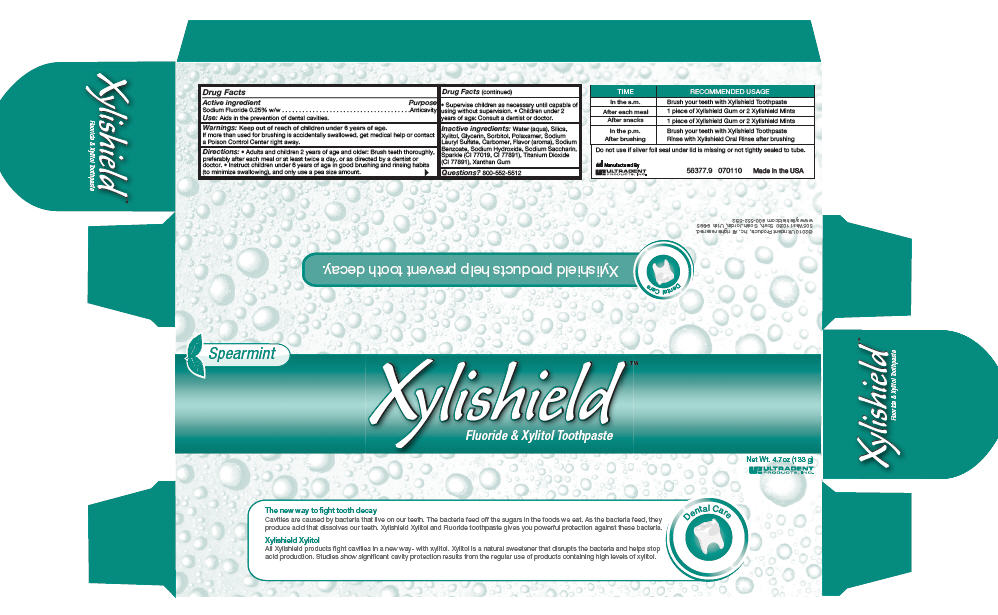
Xylishield
Fluoride & Xylitol Toothpaste
Directions
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing), and only use a pea size amount.
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
| XYLISHIELD
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Ultradent Products, Inc. (013369916) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultradent Products, Inc. | 013369916 | MANUFACTURE(51206-305) | |
Revised: 5/2016
Document Id: c4e789b5-5aac-4e72-95f5-fe5af665e0b6
Set id: 5adfde64-2e67-4b5a-982b-ac03c78d2a32
Version: 4
Effective Time: 20160524
Ultradent Products, Inc.