Label: MEDIC BOOSTER DIP- iodine teat dip liquid
-
NDC Code(s):
12994-001-01,
12994-001-02,
12994-001-03,
12994-001-04, view more12994-001-05, 12994-001-06
- Packager: Animal Medic Incorporated
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 30, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
USER SAFETY WARNINGS
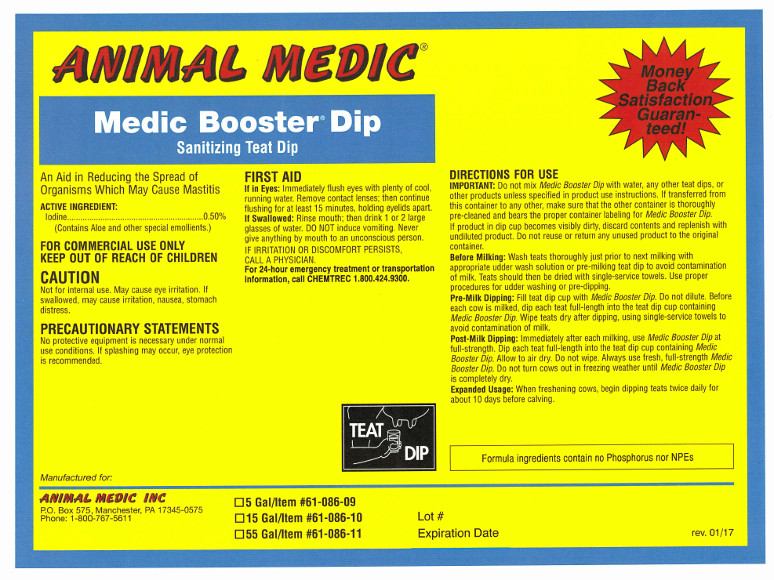
Sanitizing Teat Dip
An aid in reducing the spread of organisms which may cause mastitis.
ACTIVE INGREDIENT:
Iodine .................................................................... 0.50%
(Contains Aloe and other special emollients.)
FOR COMMERCIAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
CAUTION
Not for internal use. May cause eye irritation. If swallowed, may cause irritation, nausea, stomach distress.
PRECAUTIONARY STATEMENTS
No protective equipment is necessary under normal use conditions. If splashing may occur, eye protection is recommended.
FIRST AID
If in Eyes: Immediately flush eyes with plenty of cool, running water. Remove contact lenses; then continue flushing for at least 15 minutes, holding eyelids apart.
If Swallowed: Rinse mouth; then drink 1 or 2 glasses of water. DO NOT induce vomiting. Never give anything by mouth to an unconscious person. IF IRRITATION OR DISCOMFORT PERSISTS, CALL A PHYSICIAN.
For 24-hour emergency treatment or transportation information, call CHEMTREC 1.800.424.9300.
-
DOSAGE & ADMINISTRATION
DIRECTIONS FOR USE
IMPORTANT: Do not mix Medic Booster Dip with water, any other teat dips, or other products unless specified in product use instructions. If transferred from this container to any other, make sure that the other container is clean and bears the proper container label for Medic Booster Dip. If product in dip cup becomes visibly dirty, discard contents and replenish with undiluted product.
Do not reuse or return any unused product to the original container.Before Milking: Wash teats thoroughly just prior to next milking with appropriate udder wash solution or pre-milking teat dip to avoid contamination of milk. Teats should be dried with single-service towels. Use proper procedures for udder washing or pre-dipping.
Pre-Milk Dipping: Fill teat cup with Medic Booster Dip. Do not dilute. Before each cow is milked, dip each teat full-length into the teat cup containing Medic Booster Dip. Wipe teats dry after dipping using single-service towels to avoid contamination of milk.
Post-Milk Dipping: Immediately after each milking, use Medic Booster Dip at full strength. Dip each teat full-length into the teat cup containing Medic Booster Dip. Allow to air dry. Do not wipe. Always use fresh, full-strength Medic Booster Dip. Do not turn cows out in freezing weather until Medic Booster Dip is completely dry.
Expanded Usage: When freshening cows, begin dipping teats twice daily for about ten days before calving.
Formula ingredients contain no Phosphorus nor NPEs
- Drug Facts
-
INGREDIENTS AND APPEARANCE
MEDIC BOOSTER DIP
iodine teat dip liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:12994-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 5000 mg in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12994-001-01 3.78 L in 1 JUG 2 NDC:12994-001-02 18.9 L in 1 DRUM 3 NDC:12994-001-03 56.7 L in 1 DRUM 4 NDC:12994-001-04 113.4 L in 1 DRUM 5 NDC:12994-001-05 207.9 L in 1 DRUM 6 NDC:12994-001-06 1039.5 L in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/29/2004 Labeler - Animal Medic Incorporated (052917119)