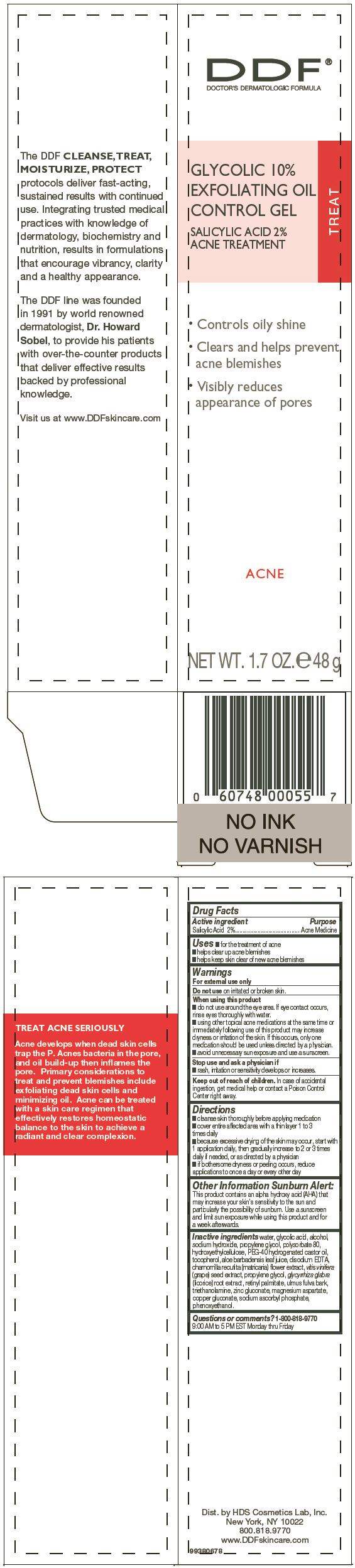
DDF GLYCOLIC EXFOLIATING- salicylic acid gel
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid 2%
Uses
- for the treatment of acne
- helps clear up acne blemishes
- helps keep skin clear of new acne blemishes
Warnings
For external use only
Do not use on irritated or broken skin.
When using this product
- do not use around the eye area. If eye contact occurs, rinse eyes thoroughly with water.
- using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a physician.
- avoid unnecessary sun exposure and use a sunscreen.
Stop use and ask a physician if
- rash, irritation or sensitivity develops or increases.
Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Directions
- cleanse skin thoroughly before applying medication
- cover entire affected area with a thin layer 1 to 3 times daily
- because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed, or as directed by a physician
- if bothersome dryness or peeling occurs, reduce applications to once a day or every other day
Other Information
Sunburn Alert
This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen and limit sun exposure while using this product and for a week afterwards.
Inactive ingredients
water, glycolic acid, alcohol, sodium hydroxide, propylene glycol, polysorbate 80, hydroxyethylcellulose, PEG-40 hydrogenated castor oil, tocopherol, aloe barbadensis leaf juice, disodium EDTA, chamomilla recutita (matricaria) flower extract, vitis vinifera (grape) seed extract, propylene glycol, glycyrrhiza glabra (licorice) root extract, retinyl palmitate, ulmus fulva bark, triethanolamine, zinc gluconate, magnesium aspartate, copper gluconate, sodium ascorbyl phosphate, phenoxyethanol.
Questions or comments?
1-800-818-9770 9:00 AM to 5 PM EST Monday thru Friday
Dist. by HDS Cosmetics Lab, Inc.
New York, NY 10022
PRINCIPAL DISPLAY PANEL - 48 g Bottle Carton
DDF®
DOCTOR'S DERMATOLOGIC FORMULA
GLYCOLIC 10%
EXFOLIATING OIL
CONTROL GEL
SALICYLIC ACID 2%
ACNE TREATMENT
TREAT
- Controls oily shine
- Clears and helps prevent
acne blemishes
- Visibly reduces
appearance of pores
ACNE
NET WT. 1.7 OZ. e 48 g