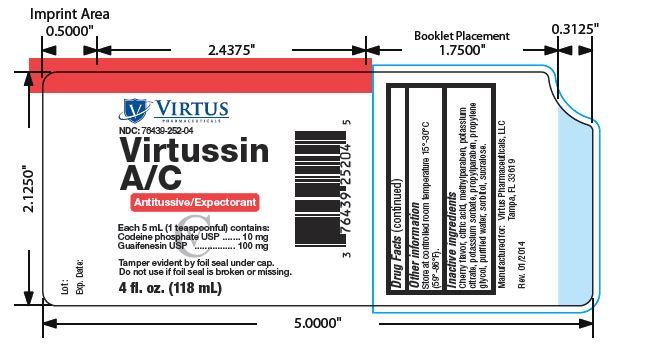
VIRTUSSIN AC- codeine phosphate and guaifenesin liquid
Virtus Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Virtussin A/C
Uses
- temporarily relieves
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- your cough to help you sleep
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and makes cough more productive.
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
When using this product
- giving a higher dose than recommended by a doctor could result in serious side effects for your child. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
- may cause or aggravate constipation
Directions
- do not exceed 6 doses in 24 hours.
| Adults and children 12 years of age and over: | 2 tsp (10 mL) every 4 hours, or as directed by a doctor. |
| Children 6 to under 12 years of age: | 1 tsp (5 mL) every 4 hours, or as directed by a doctor. |
| Children under 6 years of age: | Consult a doctor. |
| VIRTUSSIN AC
codeine phosphate and guaifenesin liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Virtus Pharmaceuticals LLC (969483143) |
Revised: 9/2017
Document Id: 58e223b2-d703-4cac-a1c1-974a54f76fe3
Set id: 58aaee70-a0dc-495b-8bfc-80d7ee7ac622
Version: 2
Effective Time: 20170920
Virtus Pharmaceuticals LLC