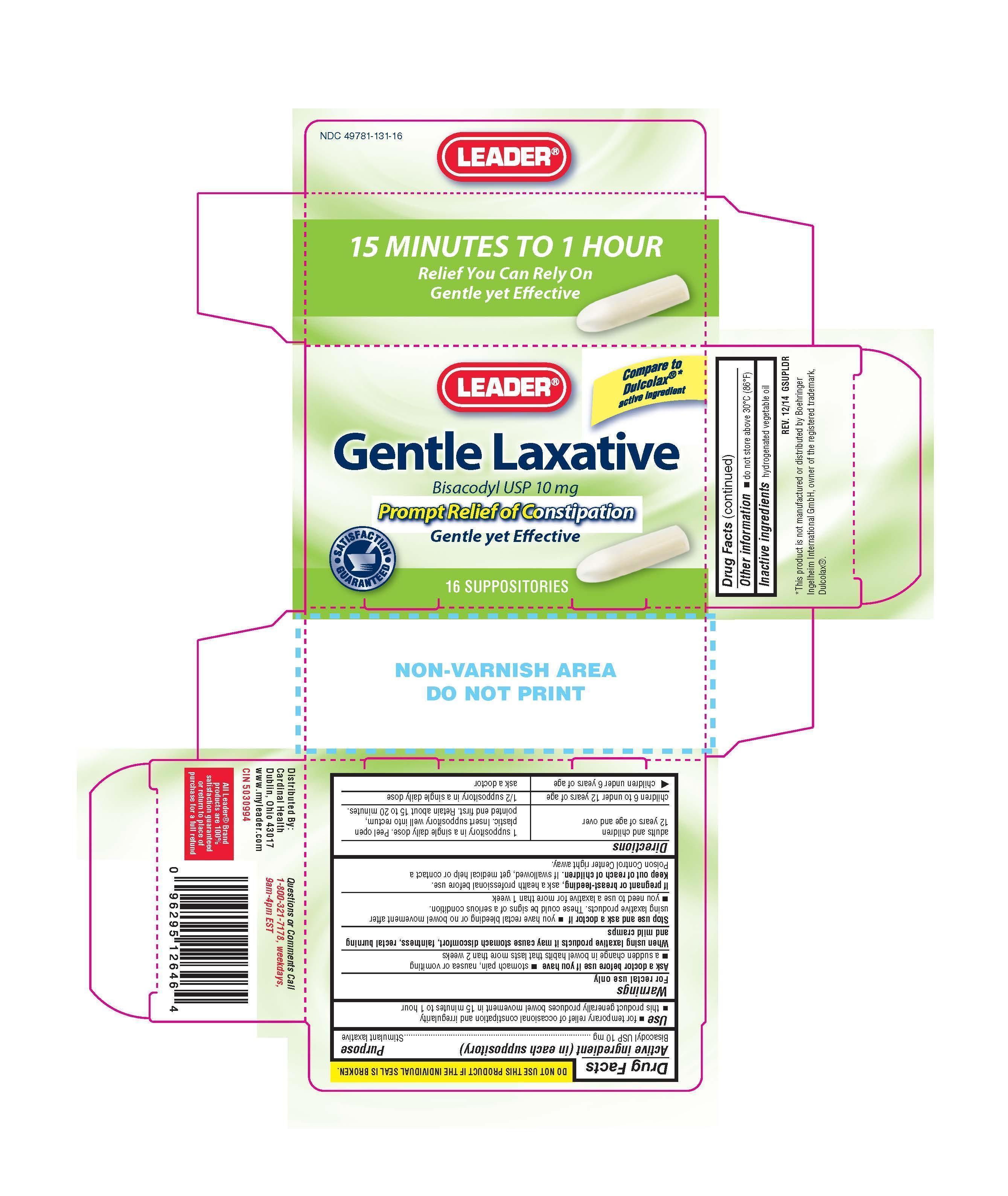
LEADER GENTLE LAXATIVE
- bisacodyl suppository
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Uses
- For relief of occasional constipation and irregularity
- -This product generally produces bowel movement in 15 minutes to 1 hour
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that persists over a period of two weeks
Stop use and ask a doctor if
- if you have rectal bleeding or fail to have bowel movement after using a laxative. This may indicate a serious condition
- you need to use a laxative for more than 1 week
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older Children 6 to under 12 years Children under 6
One suppository once daily 1/2 suppository once daily Ask doctor.
-Detach one suppository from the strip and remove from foil - Carefully insert one suppositry well into the rectum
-Do not use more than once per day
| LEADER GENTLE LAXATIVE
bisacodyl suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
| Registrant - Reese Pharmaceutical Co (004172052) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Reese Pharmaceutical Co | 004172052 | relabel(49781-131) , repack(49781-131) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biopharm | 801652546 | manufacture(49781-131) | |