Label: CLEARPREP FX- salicylic acid gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 68327-006-01, 68327-006-02, 68327-006-03 - Packager: Cover FX Skin Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Section
- Purpose Section
- Indications and Uses Section
-
Warning Section
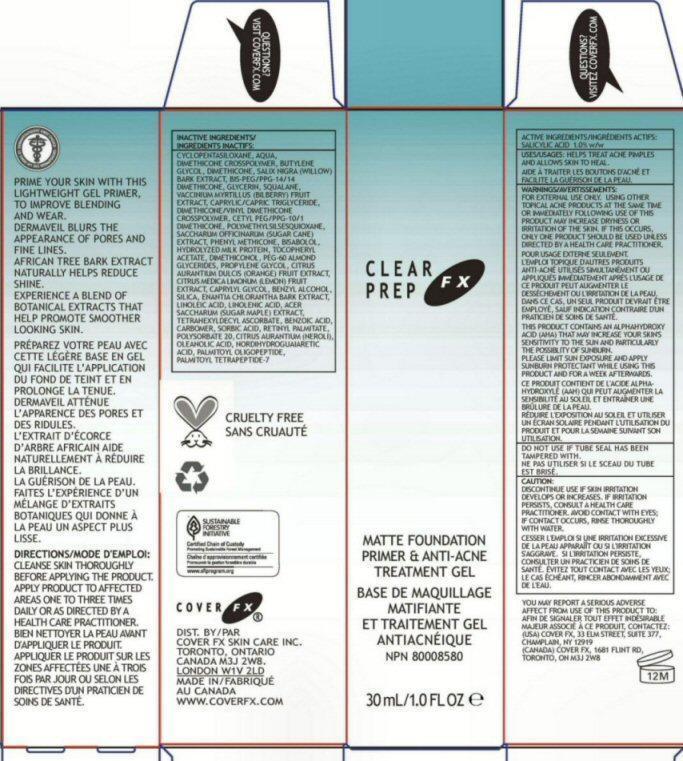
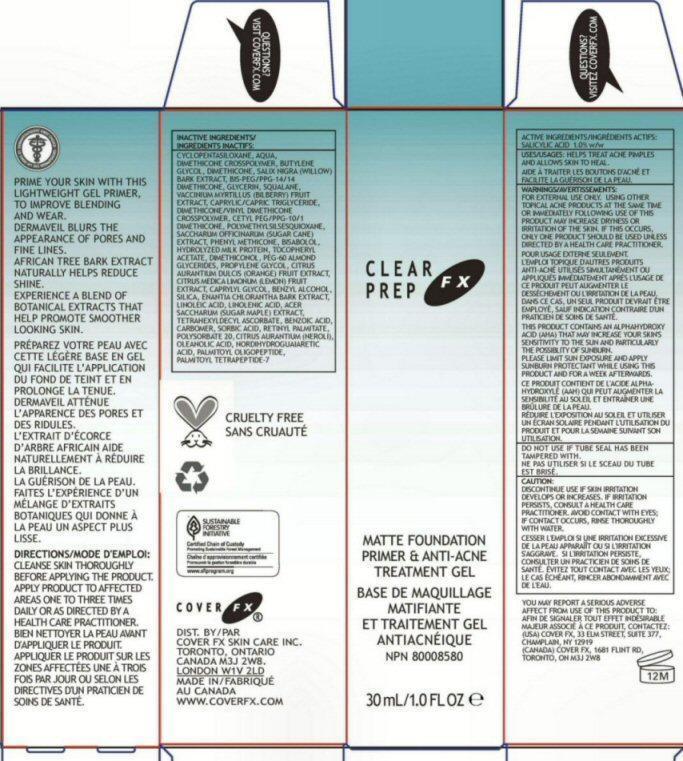
WARNINGS: FOR EXTERNAL USE ONLY. USING OTHER TOPICAL ACNE PRODUCTS AT THE SAME TIME OR IMMEDIATELY FOLLOWING USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS, ONLY ONE PRODUCT SHOULD BE USED UNLESS DIRECTED BY A HEALTH CARE PRACTITIONER.
THIS PRODUCT CONTAINS AN ALPHAHYDROXY ACID (AHA) THAT MAY INCREASE YOUR SKINS SENSITIVITY TO THE SUN AND PARTICULARLY THE POSSIBILITY OF SUNBURN. PLEASE LIMIT SUN EXPOSURE AND APPLY SUNBURN PROTECTANT WHILE USING THIS PRODUCT AND FOR A WEEK AFTERWARDS.
DO NOT USE IF TUBE SEAL HAS BEEN TAMPERED WITH.
- Caution Section
- Report Adverse Section
-
Directions Section
PRIME YOUR SIN WITH THIS LIGHTWEIGHT GEL PRIMER, TO IMPROVE BLENDING AND WEAR. DERMAVEIL BLURS THE APPEARANCE OF PORES AND FINE LINES. AFRICAN TREE BARK EXTRACT NATURALLY HELPS REDUCE SHINE. EXPERIENCE A BLEND OF BOTANICAL EXTRACTS THAT HELP PROMOTE SMOOTHER LOOKING SKIN.
DIRECTIONS: CLEANSE SKIN THOROUGHLY BEFORE APPLYING THE PRODUCT. APPLY PRODUCT TO AFFECTED AREAS ONE TO THREE TIMES DAILY OR AS DIRECTED BY A HEALTH CARE PRACTITIONER.
-
Inactive Ingredient Section
INACTIVE INGREDIENTS: CYCLOPENTASILOXANE AQUA DIMETHICONE CROSSPOLYMER BUTYLENE GLYCOL DIMETHICONE SALIX NIGRA (WILLOW) BARK EXTRACT BIS-PEG/PPG-14/14 DIMETHICONE GLYCERIN SQUALANE VACCINIUM MYRTILLUS (BILBERRY) FRUIT EXTRACT CAPRYLIC/CAPRIC TRIGLYCERIDE DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER CETYL PEG/PPG-10/1 DIMETHICONE POLYMETHYLSILSESQUIOXANE SACCHARUM OFFICINARUM (SUGER CANE) EXTRACT PHENYL METHICONE BISABOLOL HYDROLYZED MILK PROTEIN TOCOPHERYL ACETATE DIMETHICONOL PEG-60 ALMOND GLYCERIDES PROPYLENE GLYCOL CITRUS AURANTIUM DULCIS (ORANGE) FRUIT EXTRACT CITRUS MEDICA LIMONUM (LEMON) FRUIT EXTRACT CAPRYLYL GLYCOL BENZYL ALCOHOL SILICA ENANTIA CHLORANTHA BARK EXTRACT LINOLEIC ACID LINOLENIC ACID ACER SACCHARUM (SUGAR MAPLE) EXTRACT TETRAHEXYLDECYL ASCORBATE BENZOIC ACID CARBOMER SORBIC ACID RETINYL PALMITATE POLYSORBATE 20 CITRUS AURANTIUM (NEROLI) OIL OLEANOLIC ACID NORDIHYDROGUAIARECTIC ACID PALMITOYL OLIGOPEPTIDE PALMITOYL TETRAPEPTIDE-7
- Keep Out of Reach of Children Section
- Label Section
-
INGREDIENTS AND APPEARANCE
CLEARPREP FX
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68327-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 100 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) DIMETHICONE/DIENE DIMETHICONE CROSSPOLYMER (UNII: RSA9I561OK) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) SALIX NIGRA BARK (UNII: QU52J3A5B3) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) BILBERRY (UNII: 9P2U39H18W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SUGARCANE (UNII: 81H2R5AOH3) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LEVOMENOL (UNII: 24WE03BX2T) CASEIN (UNII: 48268V50D5) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ORANGE (UNII: 5EVU04N5QU) LEMON (UNII: 24RS0A988O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BENZYL ALCOHOL (UNII: LKG8494WBH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANNICKIA CHLORANTHA BARK (UNII: H70115MP4A) LINOLEIC ACID (UNII: 9KJL21T0QJ) LINOLENIC ACID (UNII: 0RBV727H71) ACER SACCHARUM BARK/SAP (UNII: Z120VL0KAC) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) BENZOIC ACID (UNII: 8SKN0B0MIM) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SORBIC ACID (UNII: X045WJ989B) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) POLYSORBATE 20 (UNII: 7T1F30V5YH) CITRUS AURANTIUM FLOWER OIL (UNII: D4BGE91OXH) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) MASOPROCOL (UNII: 7BO8G1BYQU) PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68327-006-01 30 mL in 1 TUBE 2 NDC:68327-006-02 15 mL in 1 TUBE 3 NDC:68327-006-03 7 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/01/2011 Labeler - Cover FX Skin Care, Inc. (202908021) Registrant - Cover FX Skin Care, Inc. (202908021)