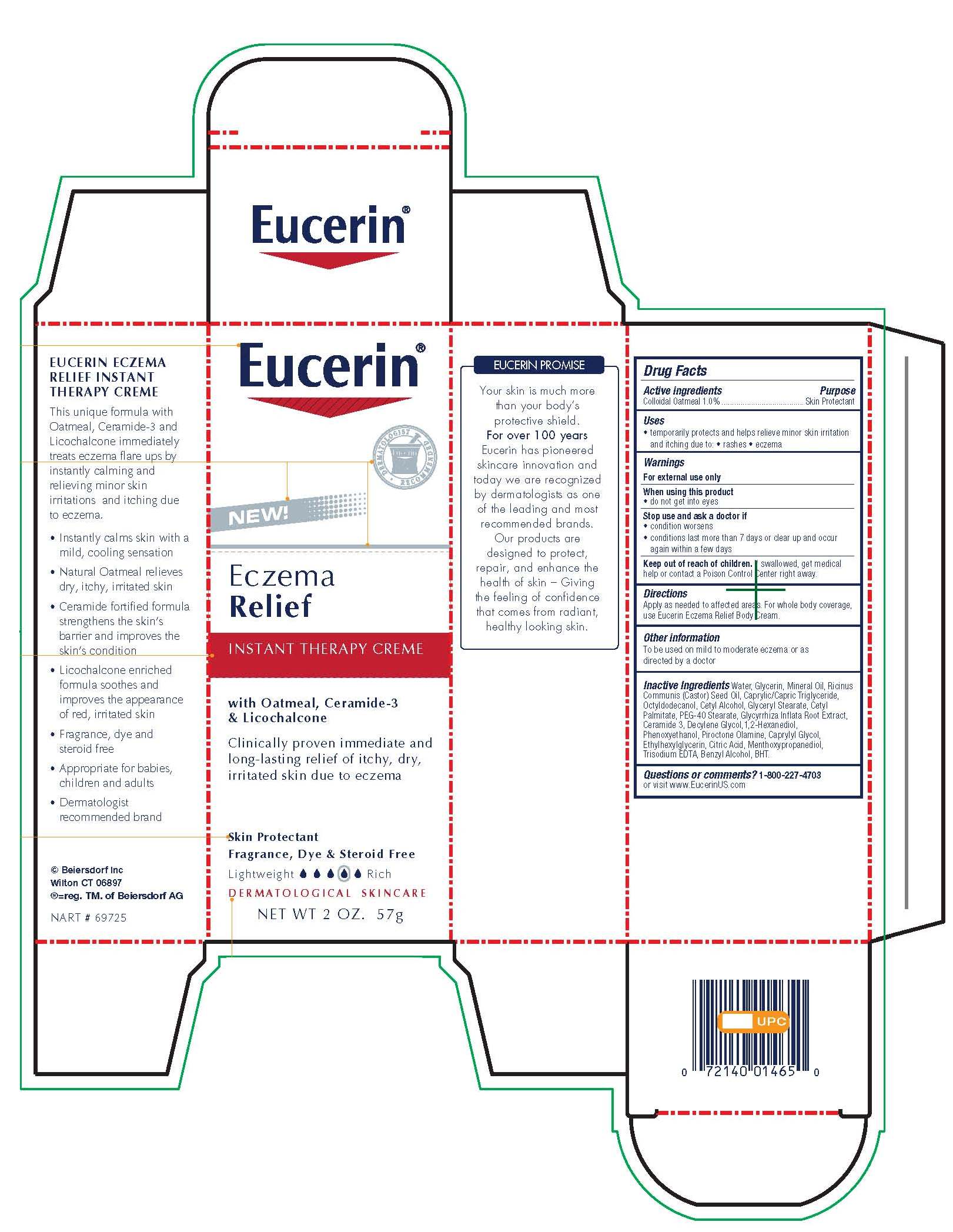
EUCERIN ECZEMA RELIEF INSTANT THERAPY- oatmeal cream
Beiersdorf Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Eucerin Eczema Relief Instant Therapy
Stop use and ask a doctor if
condition worsens
symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or
contact a Poison Control Center right away.
Directions
Apply as needed to affected areas. For whole body coverage, use Eucerin Eczema Relief Body Creme.
Inactive Ingredients
Water, Glycerin, Mineral Oil, Ricinus Communis (Castor) Seed Oil,
Caprylic/Capric Triglyceride, Octyldodecanol, Cetyl Alcohol, Glyceryl Stearate,
Cetyl Palmitate, PEG-40 Stearate, Glycyrrhiza Inflata
Root Extract, Ceramide 3, Decylene Glycol, 1,2-Hexanediol, Phenoxyethanol, Piroctone
Olamine, Caprylyl Glycol, Ethylhexylglycerin, Citric Acid, Menthoxypropanediol,
Trisodium EDTA, Benzyl Alcohol, BHT.
.
| EUCERIN ECZEMA RELIEF INSTANT THERAPY
oatmeal cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Beiersdorf Inc (001177906) |