Label: VAGICAINE MAXIMUM STRENGTH- benzocaine, resorcinol cream
- NDC Code(s): 41250-142-64
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 28, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
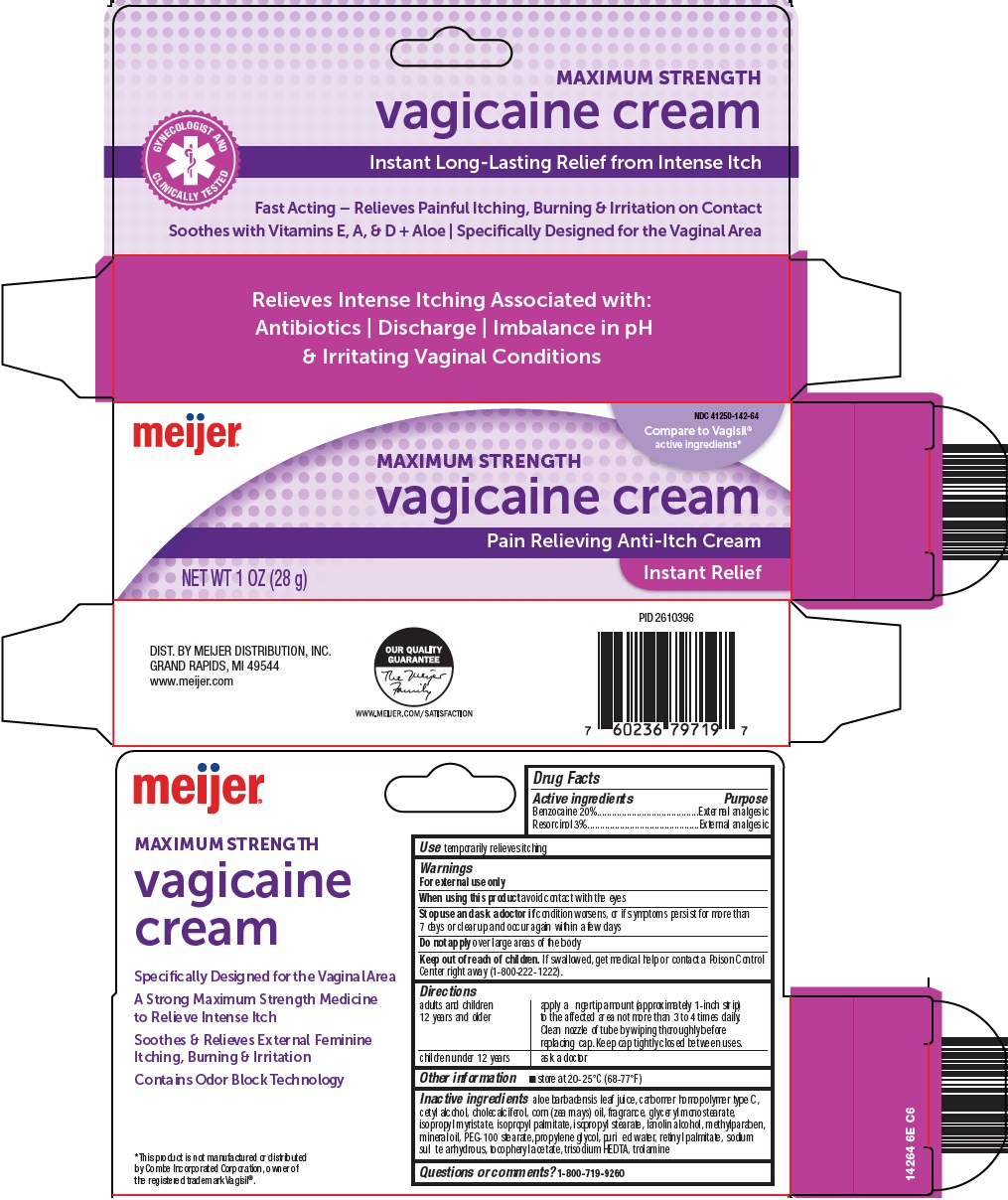
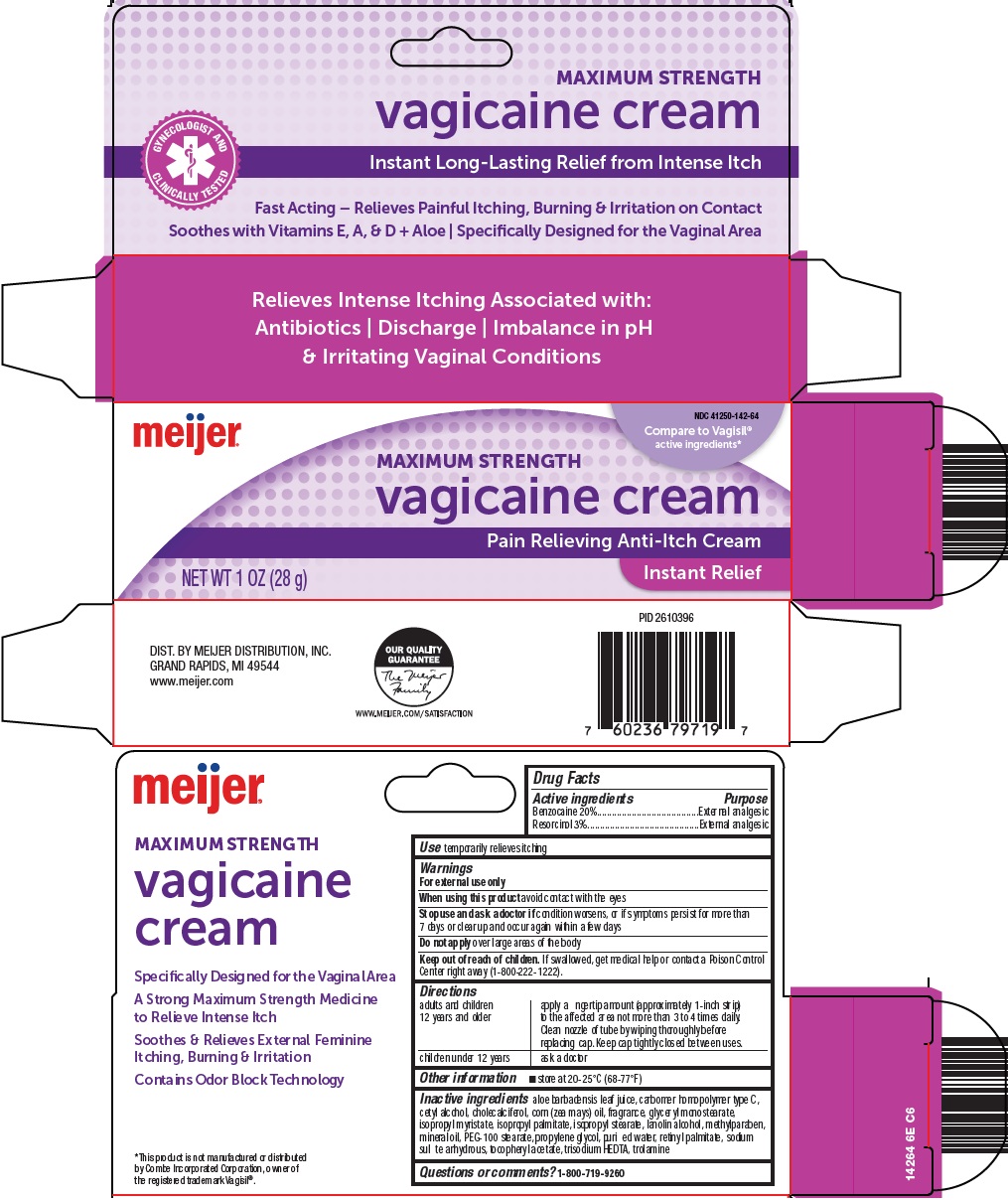
aloe barbadensis leaf juice, carbomer homopolymer type C, cetyl alcohol, cholecalciferol, corn (zea mays) oil, fragrance, glyceryl monostearate, isopropyl myristate, isopropyl palmitate, isopropyl stearate, lanolin alcohol, methylparaben, mineral oil, PEG-100 stearate, propylene glycol, purified water, retinyl palmitate, sodium sulfite anhydrous, tocopheryl acetate, trisodium HEDTA, trolamine
- Questions or comments?
-
Principal Display Panel
MAXIMUM STRENGTH
vagicaine cream
GYNECOLOGIST AND CLINICALLY TESTED
Instant Long-Lasting Relief from Intense Itch
Fast Acting – Relieves Painful Itching, Burning & Irritation on Contact
Soothes with Viatmins E, A, & D + Aloe | Specifically Designed for the Vaginal Area
Compare to Vagisil® active ingredients
MAXIMUM STRENGTH
vagicaine cream
Pain Relieving Anti-Itch Cream
NET WT. 1 OZ (28 g)
Instant Relief
-
INGREDIENTS AND APPEARANCE
VAGICAINE MAXIMUM STRENGTH
benzocaine, resorcinol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 3 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CETYL ALCOHOL (UNII: 936JST6JCN) CHOLECALCIFEROL (UNII: 1C6V77QF41) CORN OIL (UNII: 8470G57WFM) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) ISOPROPYL STEARATE (UNII: 43253ZW1MZ) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SODIUM SULFITE (UNII: VTK01UQK3G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRISODIUM HEDTA (UNII: K3E0U7O8KI) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-142-64 1 in 1 CARTON 05/15/2009 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/15/2009 Labeler - Meijer Distribution Inc (006959555)