Label: ALBURX (albumin- human solution
- NDC Code(s): 44206-310-25, 44206-310-50, 44206-310-90, 44206-310-91
- Packager: CSL Behring AG
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
ALBURX® 5, Albumin (Human) 5% solution is a sterile aqueous solution for intravenous administration containing the albumin component of human blood. The solution is clear and slightly viscous; it is almost colorless, or yellow, amber, or green.
This product is prepared from the plasma of US donors. The product has been produced by alcohol fractionation and has been heated for 10 hours at 60°C for inactivation of infectious agents. The results of virus validation studies have shown that the manufacturing process, particularly alcohol fractionation, eliminates enveloped and non-enveloped viruses. Additionally, heat treatment at 60°C for a period of 10 hours efficiently inactivates viruses. The solution contains 0.14 M (3.2 mg/mL) sodium. The aluminum content is ≤ 200 mcg/L and the potassium content is ≤ 0.002 M. The solution is stabilized with 0.004 M sodium N-acetyltryptophanate and 0.004 M sodium caprylate. The solution contains no preservative.
-
CLINICAL PHARMACOLOGY10,16
ALBURX® 5, Albumin (Human) 5% solution should not be used as an intravenous nutrient because of the slow breakdown and relatively unfavorable composition of the albumin molecule with respect to its content of essential amino acids. Oral provision of proteins or an intravenous regimen providing adequate calories and a suitable amino acid mixture are the methods of choice for the treatment of protein malnutrition as such, though they do not permit the rapid correction of hypoproteinemia.
The binding properties of albumin may, in special circumstances, provide an indication for its clinical use. For such purposes, however, an Albumin (Human) 25% solution should be used.
The colloid osmotic or oncotic properties of albumin constitute the predominant reason for its clinical use. The rationale for this is the Starling concept of the capillary balance of hydrostatic and oncotic pressure gradients across the capillary walls as the determinant of the fluid – i.e. volume – distribution between the intravascular and the interstitial compartment.15 The basic indication for the use of ALBURX® 5, Albumin (Human) 5% solution is therefore a plasma or blood volume deficit. The 5% concentration is approximately isotonic and isooncotic with normal human plasma. The effective colloid osmotic pressure of the serum proteins depends very largely on the relatively small and numerous albumin molecules, which therefore play a decisive role in the maintenance of the circulating plasma volume.
-
INDICATIONS AND USAGE
Shock
The definitive treatment of major hemorrhage is the transfusion of red blood cells restoring a normal oxygen transport capacity of the blood. Since, however, the life-threatening event in major hemorrhage is the loss of blood volume and not the erythrocyte deficit, the blood volume can, as an emergency measure, be supported by ALBURX® 5, Albumin (Human) 5% solution or another rapidly acting plasma substitute if blood is not immediately available. This will restore cardiac output and abolish circulatory failure with tissue anoxia. Though a four- to fivefold volume of crystalloids may be equally effective, their administration takes more time and creates a general overload with sodium and water. In the presence of dehydration, electrolyte solutions such as Ringer's lactate should be administered in conjunction with albumin.
Burns
Apart from damage to the respiratory tract, the development of burn shock is the most life-threatening event in the immediate care of the burned patient. Therapy during the first 24 hours is directed at the administration of large volumes of crystalloid solutions and lesser amounts of ALBURX® 5, Albumin (Human) 5% solution to maintain an adequate plasma volume and protein (colloid) content. For continuation of therapy beyond 24 hours, larger amounts of ALBURX® 5, Albumin (Human) 5% solution and lesser amounts of crystalloid are generally used.16 An optimum regimen for the use of Albumin (Human), electrolytes, and fluid in the early treatment of burns has, however, not yet been established.
Pancreatitis and peritonitis
ALBURX® 5, Albumin (Human) 5% solution is useful in the early therapy of shock associated with acute hemorrhagic pancreatitis and peritonitis. It has been found that the correction of the blood volume deficit and adequate fluid therapy are mandatory in the acute stage of pancreatitis and peritonitis when there is loss of fluid into the peritoneal cavity or the retroperitoneal space.1
Postoperative albumin loss
It is now recognized that intra-operative damage to capillary walls by blunt handling and sharp dissection of tissues leads to substantial postoperative losses of circulating albumin, over and above those due to bleeding. Forty to eighty percent of the intravascular albumin mass may thus be lost after radical dissections for malignant disease, surgery of the colon and rectum, and reconstructive procedures involving the aorta and major iliac vessels.4,7,14 ALBURX® 5, Albumin (Human) 5% solution is a suitable agent for the correction of the resultant loss of plasma volume and in this situation may be superior to electrolyte solutions in maintaining early postoperative pulmonary function.13 However, temporary redistribution of protein is usually not an indication for Albumin (Human).
Hypoproteinemia with an oncotic deficit
In subacute or chronic hypoproteinemia, efforts should always be made to determine the underlying cause and to improve circulating protein levels by dietary means. Most commonly, such states are due to protein-calorie malnutrition, defective absorption in gastrointestinal disorders, faulty albumin synthesis in chronic hepatic failure, increased protein catabolism postoperatively or with sepsis, and abnormal renal losses of albumin with chronic kidney disease. In all these situations, the circulating plasma volume is usually maintained by the renal retention of sodium and water, but this is associated with tissue edema due to the hypoalbuminemia with an oncotic deficit. The cutaneous edema lowers the oxygen tension of wounds and may thus impair the healing process3, and the oncotic deficit favors the development of interstitial pulmonary edema2 and the intestinal accumulation of fluids, which may progress to a paralytic ileus.7 Though relief of the basic pathology is the definitive mode of therapy for the restoration of the plasma protein content, this process takes time to become effective, and the rapid correction of an oncotic deficit by the administration of Albumin (Human) may be indicated. For this purpose, however, Albumin (Human) 25% is preferable, possibly in conjunction with a diuretic.12 It is emphasized that whereas Albumin (Human) may be needed to treat the aforementioned acute complications of chronic hypoproteinemia, it is not indicated for treatment of the chronic condition itself.
-
CONTRAINDICATIONS
The use of ALBURX® 5, Albumin (Human) 5% solution is contraindicated in patients with a history of an incompatibility reaction to such preparations (see ADVERSE REACTIONS). In addition, ALBURX® 5 may be contraindicated in patients with cardiac failure or severe anemia because of the risk of acute circulatory overload.
-
WARNINGS
ALBURX® 5, Albumin (Human) 5% solution is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, that can cause disease. The risk that such products will transmit an infectious agent has been extremely reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses through alcohol fractionation and through heat treatment of the product in the final container for 10 hours at 60ºC. Despite these measures, such products can still potentially transmit disease. A theoretical risk for transmission of Creutzfeldt-Jakob Disease (CJD) is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for Albumin (Human). There is also the possibility that unknown infectious agents may be present in such products. All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to CSL Behring Pharmacovigilance Department at 1-866-915-6958.
The physician should discuss the risks and benefits of this product with the patient.
Turbid solutions must not be used. Do not begin administration more than 4 hours after introduction of the administration set.
-
PRECAUTIONS
Adequate precautions should be taken against circulatory overload (see DOSAGE AND ADMINISTRATION). Acute pulmonary edema is seen in 3 to 4 percent of patients resuscitated from severe shock, but this is neither related to any particular type of resuscitative fluid, nor is it necessarily due to circulatory overload.11 Helpful measures are pulmonary auscultation and if possible measurement of the central venous pressure. Special caution is indicated in patients with stabilized chronic anemia or renal insufficiency.
PREGNANCY
Animal reproduction studies have not been conducted with ALBURX® 5, Albumin (Human) 5% solution. It is also not known whether ALBURX® 5 can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. ALBURX® 5 should be given to a pregnant woman only if clearly needed. There is, however, no evidence for any contraindication to the use of ALBURX® 5 specifically associated with reproduction, pregnancy or the fetus.
Use an intravenous infusion set suitable for the infusion of blood and blood products.
-
ADVERSE REACTIONS
Since ALBURX® 5, Albumin (Human) 5% solution is sterile when coming from the manufacturer, bacterial contamination with the risk of post-infusion septicemia can only occur if the container has been damaged or following puncture of the rubber cap (see WARNINGS).
Though very rare, non-septic incompatibility reactions including nausea, chills, fever, urticaria, headache and hypotension following the administration of albumin-containing preparations have occasionally been observed.6,8,9,16 A favorable response to the intravenous administration of 50 to 100 mg of prednisolone was recorded.9 Severe allergic reactions such as anaphylactic shock have been reported.
-
DOSAGE AND ADMINISTRATION
ALBURX® 5, Albumin (Human) 5% solution must be administered intravenously. The venipuncture site should not be infected or traumatized, and should be prepared with standard aseptic technique. The solution is compatible with whole blood or packed red cells as well as the usual electrolyte and carbohydrate solutions intended for intravenous use. By contrast, it should not be mixed with protein hydrolysates, amino acid mixtures, or solutions containing alcohol. It is ready for use as contained in the bottle and may be given without regard to the blood group of the recipient.
Upon administration of ALBURX® 5, Albumin (Human) 5% solution, there is a rapid increase of the plasma volume about equal to the volume infused. The initial dose for adults is 250 or 500 mL. The rate of infusion and the total volume administered are determined by the condition and response of the patient. A rate of 1–2 mL per minute is usually suitable in the absence of overt shock, whereas the capacity of the administration set is the only limit in the exsanguinated patient.
During resuscitation, constant monitoring of the patient provides the guidelines for treatment.
For children, a dose of 22 to 33 mL per kilogram body weight is usually adequate and close surveillance of the young patient is essential. Since patients – notably those with sepsis or severe multiple injuries – often need a circulating blood volume exceeding the prediction derived from their body weight, treatment should always be guided by the hemodynamic response and not by blood volume calculations or measurements.5
ALBURX® 5, Albumin (Human) 5% is clear and slightly viscous; it is almost colorless, or yellow, amber, or green. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use solution that is turbid or contains particulate matter. Partially used bottles must be discarded.
-
HOW SUPPLIED
ALBURX® 5 is supplied as a 5% solution (50 g/L).
Each product presentation includes a package insert and the following components listed in Table 1 below.
Table 1. How Supplied Presentation Carton
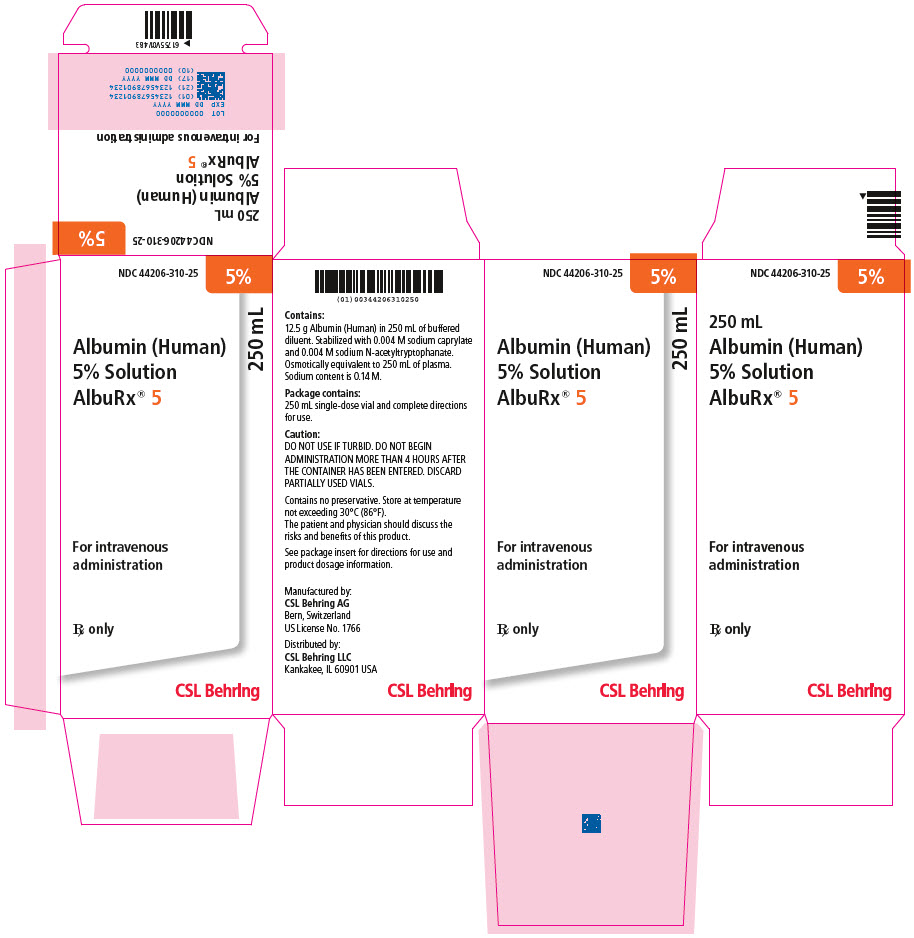
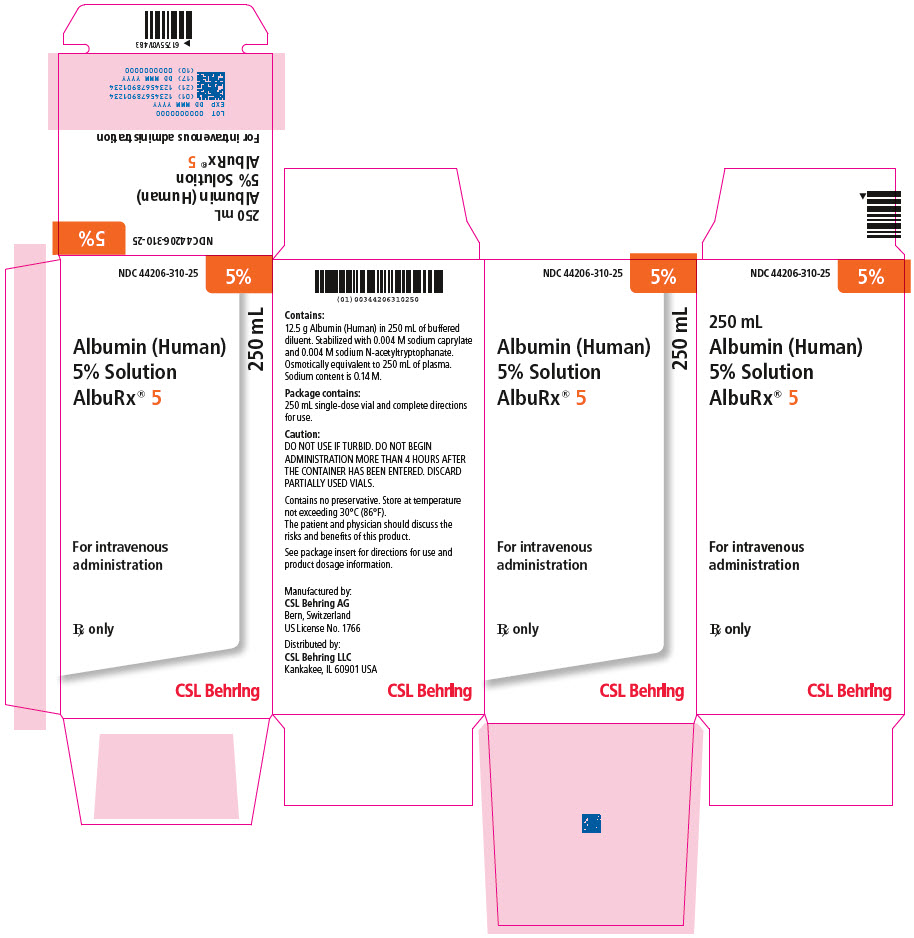
NDC NumberComponents 250 mL 44206-310-25 One single-dose vial containing 12.5 grams of albumin
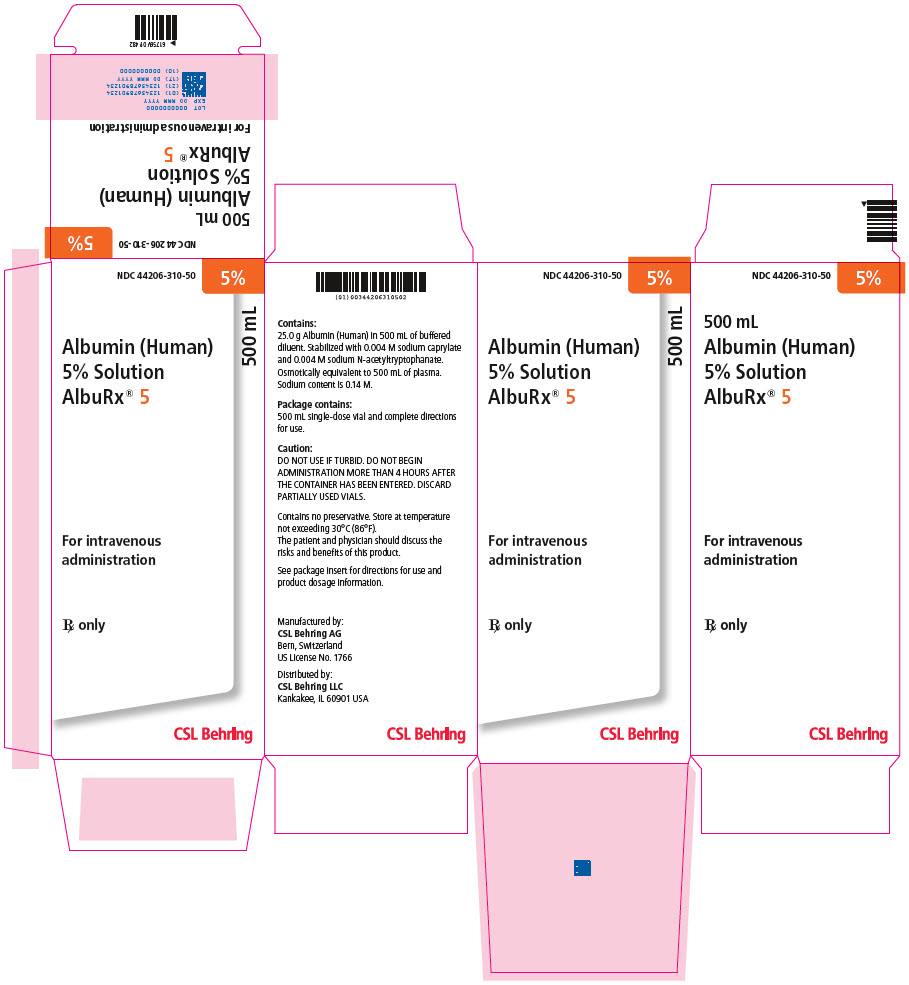
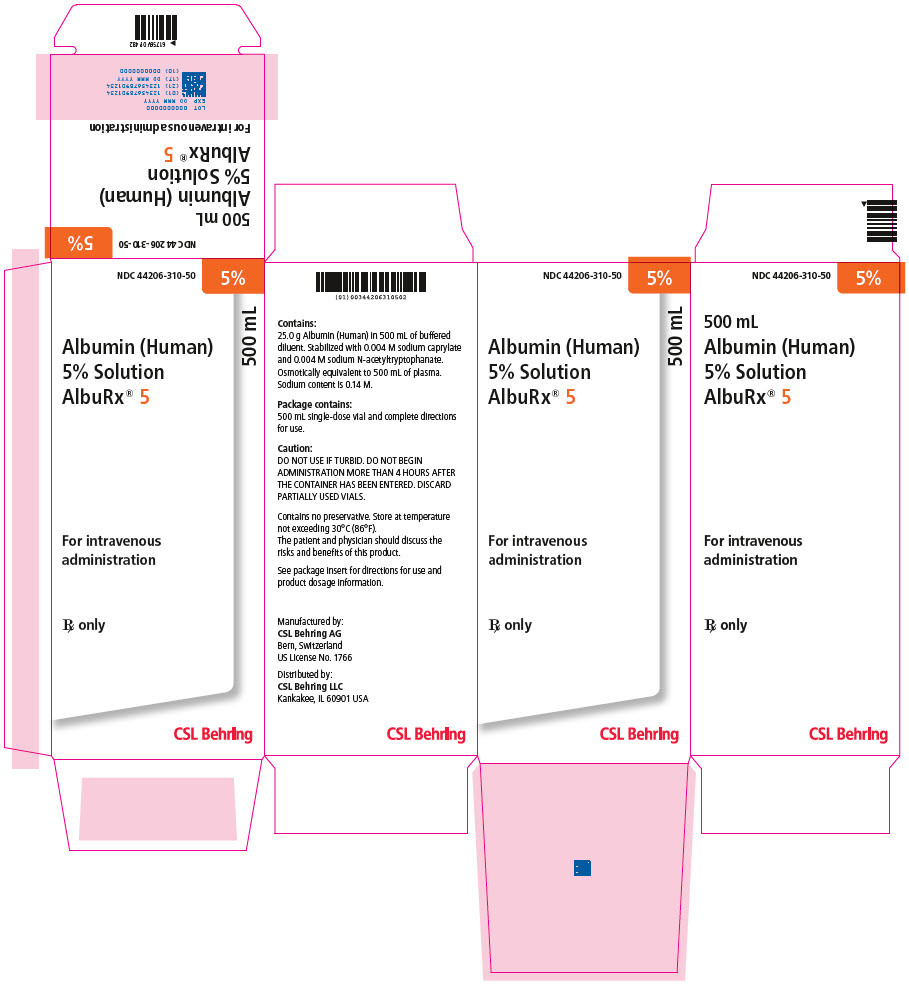
[NDC 44206-310-90]500 mL 44206-310-50 One single-dose vial containing 25 grams of albumin
[NDC 44206-310-91] - STORAGE
-
REFERENCES
- Clowes GHA Jr, Vucinic M, Weidner MG. Ann. Surg. 1966;163:866.
- Gaar KA Jr, Taylor AE, Owens LJ, Guyton AC. Amer. J. Physiol. 1967;213:79.
- Heughan C, Niinikoski J, Hunt TK. Surg. Gynec. Obstet. 1972;135:257.
- Hoye RC, Paulson DF, Ketcham AS. Surg. Gynec. Obstet. 1970;131:943.
- Kinney JM, Egdahl RH, Zuidema GD. Manual of Preoperative and Postoperative Care, American College of Surgeons. Philadelphia: W. B. Saunders Co: 1971.
- Lowenstein E. In: Sgouris JT, René A, ed. Proceedings of the Workshop on Albumin, February 12–13. Bethesda, Maryland 20014: DHEW Publication NIH 76-925, NHLI; p. 302, 1975.
- Moss G. Surg. Forum. 1967;18:333.
- Ring J, Messmer K. Lancet. 1977;1:466.
- Ring J, Seifert J, Lob G, Coulin K, Brendel W. Klin. Wschr. 1974;52:595.
- Sgouris JT, René A, ed. Proceedings of the Workshop on Albumin, February 12–13. Bethesda, Maryland 20014: DHEW Publication NIH 76-925, NHLI; 1975.
- Simmons RL, Heisterkamp CA, Collins JA, Bredenberg CE, Martin AM. J. Trauma. 1969;9:760.
- Skillman JJ, Parikh BM, Tanenbaum BJ. Amer. J. Surg. 1970;119:440.
- Skillman JJ, Restall DS, Salzman EW. Surgery. 1975;78:291.
- Skillman JJ, Tanenbaum BJ. Current Topics in Surgical Research. Vol. 2. New York: Academic Press; 1970:523.
- Starling EH. J. Physiol. 1896;19:312-326.
- Tullis JL. JAMA. 1977:237:355,460.
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - 250 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 500 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
ALBURX
albumin (human) solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:44206-310 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) (ALBUMIN HUMAN - UNII:ZIF514RVZR) ALBUMIN HUMAN 12.5 g in 250 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) N-ACETYL-DL-TRYPTOPHAN SODIUM (UNII: 3EN9H0M2FX) SODIUM CAPRYLATE (UNII: 9XTM81VK2B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44206-310-25 1 in 1 CARTON 1 NDC:44206-310-90 250 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC:44206-310-50 1 in 1 CARTON 2 NDC:44206-310-91 500 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102366 01/06/2009 Labeler - CSL Behring AG (481152762) Establishment Name Address ID/FEI Business Operations CSL Behring AG 481152762 MANUFACTURE Establishment Name Address ID/FEI Business Operations CSL Behring LLC 058268293 MANUFACTURE