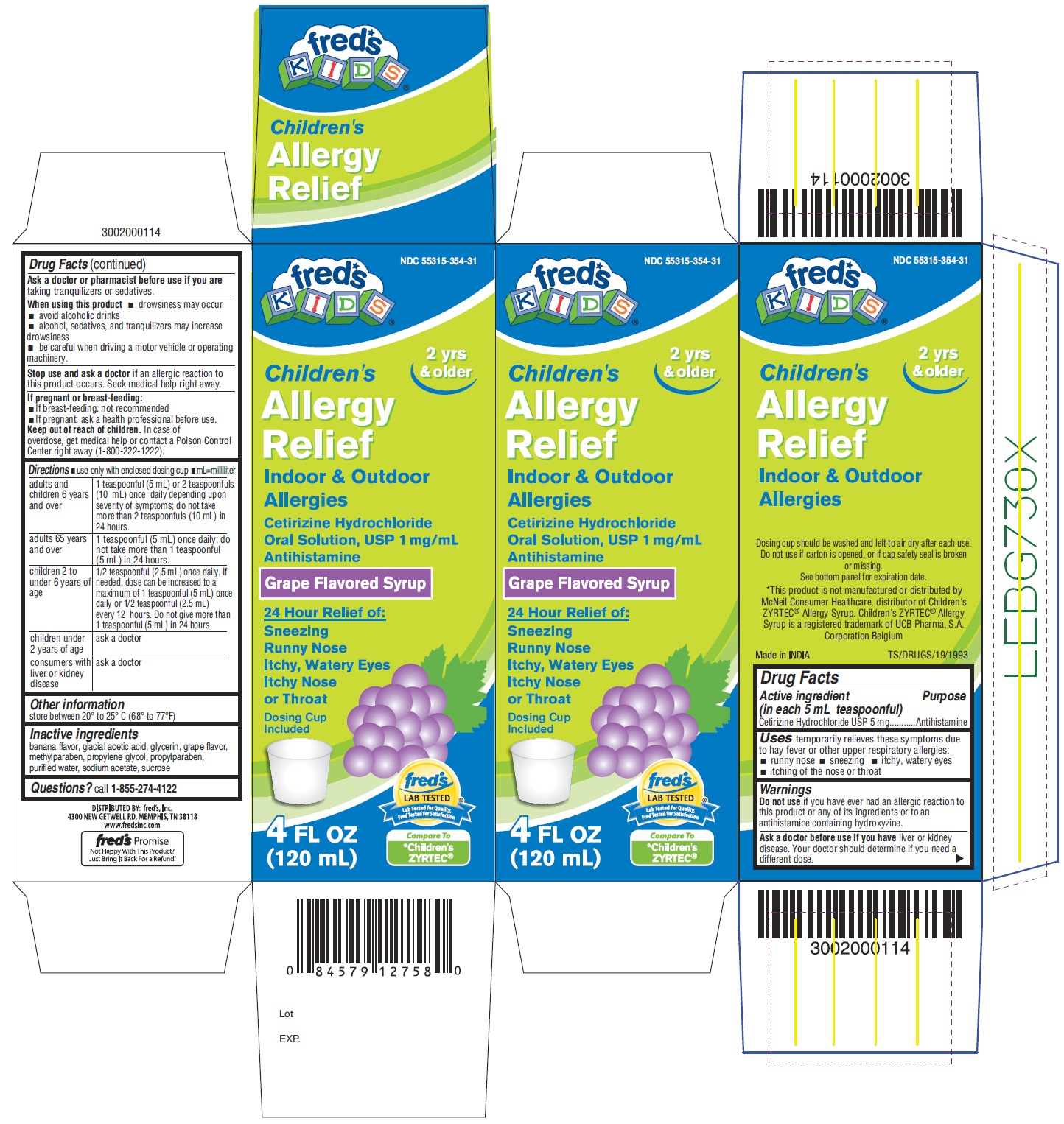
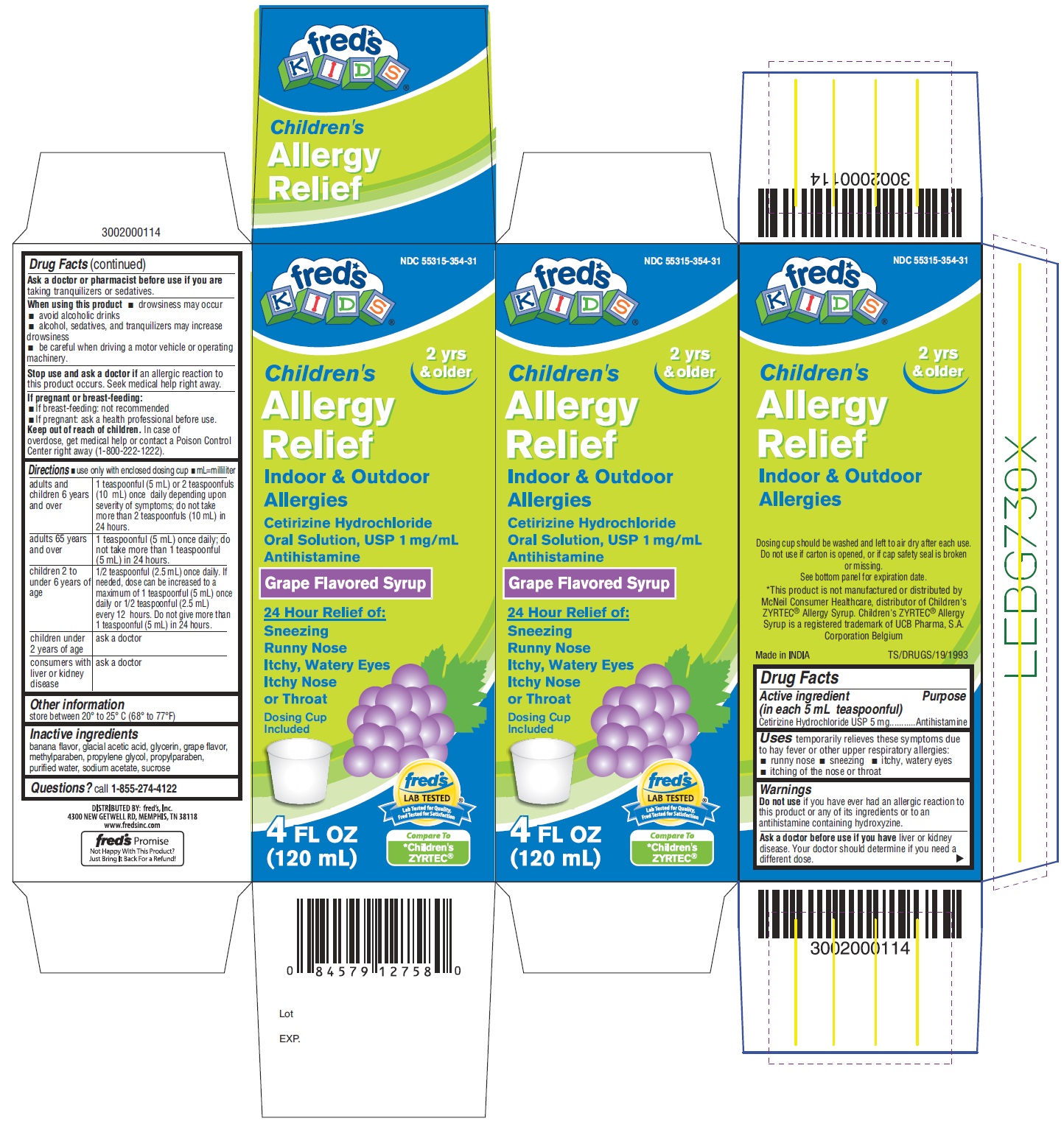
Label: CHILDRENS ALLERGY RELIEF- cetirizine hydrochloride solution
- NDC Code(s): 55315-354-31
- Packager: Fred's, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 13, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
- Warnings
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children.
-
Directions
- use only with enclosed dosing cup
- mL=milliliter
adults and children 6 years and over
1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours.
adults 65 years and over
1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours.
children 2 to under 6 years of age
1/2 teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or 1/2 teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours.
children under 2 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions?
-
PACKAGE LABEL- PRINCIPAL DISPLAY PANEL 4 FL OZ (120 mL Bottle)
NDC 55315-354-31
fred*s
KIDS®
2 yrs & older
Children's
Allergy
Relief
Indoor & Outdoor
AllergiesCetirizine Hydrochloride
Oral Solution, USP 1mg/mL
Antihistamine
Grape Flavored Syrup
24 Hour Relief of:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Nose or Throat
Dosing Cup
Included
4 FL OZ (120 mL)
Compare to
*Children's ZYRTEC®
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
cetirizine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55315-354 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM ACETATE (UNII: 4550K0SC9B) SUCROSE (UNII: C151H8M554) Product Characteristics Color YELLOW (Colorless to Pale Yellow) Score Shape Size Flavor BANANA, GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55315-354-31 1 in 1 CARTON 01/31/2015 1 120 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090750 01/31/2015 Labeler - Fred's, Inc. (005866116) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 MANUFACTURE(55315-354)