ANTISEPTIC SKIN CLEANSER- chlorhexidine gluconate solution
Cardinal Health
----------
4% Leader
Uses
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
- patient preoperative skin preparation: for the preparation of the patient's skin prior to surgery
- skin wound and general skin cleansing
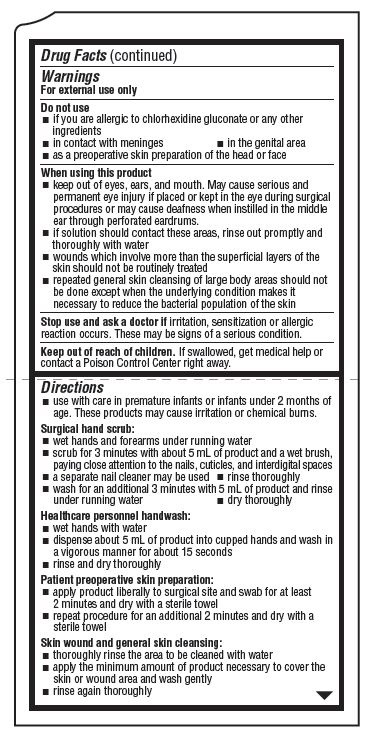
Warnings
For external use only
Do not use
- if you are allergic to chlorhexidine gluconate or any other ingredients
- in contact with meninges
- in the genital area
- as a preoperative skin preparation of the head or face
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle ear through perforated eardrums
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when the underlying condition makes it necessary to reduce the bacterial population of the skin
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms under running water
- scrub for 3 minutes with about 5 mL of product and a wet brush, paying close attention to the nails, cuticles, and interdigital spaces
- a seperate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product and rinse under running water
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 mL of product in cupped hands and wash in a vigorous manner for about 15 seconds
- rinse and dry thoroughly
Patient preoperative skin preparation:
- apply product liberally to surgical site and swab for at least 2 minutes and dry with a sterile towel
- repeat procedure for an additional 2 minutes and dry with a sterile towel
Skin wound and general skin cleansing:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
Inactive ingredients
cocamide DEA, fragrance, glucono-delta-lactone, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, PEG-75 lanolin, purified water, tridecyl alcohol.
Warning: This product contains a chemical (cocamide DEA) known to the State of California to cause cancer.
Laundering/Cleaning Instructions: Chlorhexidine gluconate skin cleansers will cause stains if used with chlorine releasing products. Rinse completely and use only non-chlorine detergents.
*This product is not manufactured or distributed by Mölnlycke Healthcare, owner of the registered trademark Hibiclens®.
Principal Display
NDC 37205-595-32
LEADER
Compare to Hibiclens active ingredient
Antiseptic Skin Cleanser
(CHLORHEXIDINE GLUCONATE 4% SOLUTION)
ANTISEPTIC / ANTIMICROBIAL SKIN CLEANSER
FOR EXTERNAL USE ONLY
DYE FREE
8 FL OZ (237 mL)
TAMPER EVIDENT: DO NOT USE IF
shrink seal neckband is broken or missing



| ANTISEPTIC SKIN CLEANSER
chlorhexidine gluconate solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
| Registrant - Xttrium Laboratories, Inc. (007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Xttrium Laboratories, Inc. | 007470579 | manufacture(37205-595) | |