Label: ULORIC- febuxostat tablet
-
NDC Code(s):
64764-677-11,
64764-677-13,
64764-677-19,
64764-677-30, view more64764-918-11, 64764-918-18, 64764-918-30, 64764-918-90
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ULORIC safely and effectively. See full prescribing information for ULORIC.
ULORIC (febuxostat) tablets, for oral use
Initial U.S. Approval: 2009WARNING: CARDIOVASCULAR DEATH
See full prescribing information for complete boxed warning.
- Gout patients with established cardiovascular (CV) disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study. (5.1)
- Consider the risks and benefits of ULORIC when deciding to prescribe or continue patients on ULORIC. ULORIC should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. (1)
INDICATIONS AND USAGE
ULORIC is a xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. (1)
Limitations of Use:
ULORIC is not recommended for the treatment of asymptomatic hyperuricemia. (1)
DOSAGE AND ADMINISTRATION
- Recommended dosage is 40 mg or 80 mg once daily. The recommended starting dosage is 40 mg once daily. For patients who do not achieve a serum uric acid (sUA) less than 6 mg/dL after 2 weeks, the recommended dosage is 80 mg once daily. (2.1)
- Patients with severe renal impairment: Limit the dosage to 40 mg once daily. (2.2, 8.6)
- Flare prophylaxis is recommended upon initiation of ULORIC. (2.4)
- Can be administered without regard to food or antacid use. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablet: 40 mg, 80 mg. (3)
CONTRAINDICATIONS
ULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine. (4)
WARNINGS AND PRECAUTIONS
- Gout Flares: An increase in gout flares is frequently observed after initiation of ULORIC. If a gout flare occurs during treatment, ULORIC need not be discontinued. Prophylactic therapy (i.e., non-steroidal anti-inflammatory drug or colchicine) upon initiation of treatment may be beneficial for up to six months. (2.4, 5.2)
- Hepatic Effects: Cases of hepatic failure, some fatal, have been reported. If liver injury is detected, promptly interrupt ULORIC and treat cause, if possible, to resolution or stabilization. Permanently discontinue ULORIC if liver injury is confirmed, and no alternate etiology can be found. (5.3)
- Serious Skin Reactions: Serious skin and hypersensitivity reactions, including Stevens-Johnson Syndrome, drug reaction with eosinophilia and systemic symptoms and toxic epidermal necrolysis have been reported in patients taking ULORIC. Discontinue ULORIC if serious skin reactions are suspected. (5.4)
ADVERSE REACTIONS
Adverse reactions in ≥ 1% of patients treated with ULORIC are liver function abnormalities, nausea, arthralgia, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant administration of ULORIC with XO substrate drugs, azathioprine or mercaptopurine could increase plasma concentrations of these drugs resulting in severe toxicity. (7)
USE IN SPECIFIC POPULATIONS
- Patients with severe hepatic impairment: No studies have been conducted in this patient population. Caution should be exercised in these patients. (8.7)
- Patients with secondary hyperuricemia (including patients being treated for Lesch-Nyhan syndrome or malignant disease, or in organ transplant recipients): ULORIC is not recommended for use as no studies have been conducted in this patient population. (8.8)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOVASCULAR DEATH
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Recommendations in Patients with Renal Impairment and Hepatic Impairment
2.3 Serum Uric Acid Level Monitoring
2.4 Recommended Prophylaxis for Gout Flares
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Death
5.2 Gout Flares
5.3 Hepatic Effects
5.4 Serious Skin Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Xanthine Oxidase Substrate Drugs
7.2 Cytotoxic Chemotherapy Drugs
7.3 In Vivo Drug Interaction Studies
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Secondary Hyperuricemia
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
14.1 Management of Hyperuricemia in Gout
14.2 Cardiovascular Safety Study
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DEATH
Gout patients with established cardiovascular (CV) disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study [see Warnings and Precautions (5.1)].
Consider the risks and benefits of ULORIC when deciding to prescribe or continue patients on ULORIC. ULORIC should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable [see Indications and Usage (1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended ULORIC dosage is 40 mg or 80 mg once daily.
The recommended starting dosage of ULORIC is 40 mg once daily. For patients who do not achieve a serum uric acid (sUA) less than 6 mg/dL after two weeks, the recommended ULORIC dosage is 80 mg once daily.
ULORIC can be taken without regard to food or antacid use [see Clinical Pharmacology (12.3)].
Concurrent prophylactic treatment with a non-steroidal anti-inflammatory drug (NSAID) or colchicine is recommended [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)].
2.2 Dosage Recommendations in Patients with Renal Impairment and Hepatic Impairment
The recommended dosage of ULORIC is limited to 40 mg once daily in patients with severe renal impairment. No dose modification is necessary when administering ULORIC in patients with mild or moderate renal impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
No dosage modification is necessary in patients with mild to moderate hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.3 Serum Uric Acid Level Monitoring
Testing for the target serum uric acid level of less than 6 mg/dL may be performed as early as two weeks after initiating ULORIC therapy.
2.4 Recommended Prophylaxis for Gout Flares
Gout flares may occur after initiation of ULORIC due to changing serum uric acid levels resulting in mobilization of urate from tissue deposits. Flare prophylaxis with a non-steroidal anti-inflammatory drug (NSAID) or colchicine is recommended upon initiation of ULORIC. Prophylactic therapy may be beneficial for up to six months [see Clinical Studies (14.1)].
If a gout flare occurs during ULORIC treatment, ULORIC need not be discontinued. The gout flare should be managed concurrently, as appropriate for the individual patient [see Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Death
In a cardiovascular (CV) outcome study, gout patients with established CV disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol. Sudden cardiac death was the most common cause of adjudicated CV deaths, 2.7% in the ULORIC group (83 of 3,098) as compared to 1.8% in the allopurinol group (56 of 3,092). ULORIC was similar to allopurinol for nonfatal myocardial infarction (MI), nonfatal stroke and unstable angina with urgent coronary revascularization [see Clinical Studies (14.2)].
Because of the increased risk of CV death, ULORIC should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable [see Indications and Usage(1)].
Consider the risks and benefits of ULORIC when deciding to prescribe or continue patients on ULORIC. Consider use of prophylactic low-dose aspirin therapy in patients with a history of CV disease. Monitor patients for the development of CV events. Inform patients about the symptoms of serious CV events and the steps to take if they occur.
5.2 Gout Flares
After initiation of ULORIC, an increase in gout flares is frequently observed. This increase is due to reduction in serum uric acid levels, resulting in mobilization of urate from tissue deposits.
In order to prevent gout flares when ULORIC is initiated, concurrent prophylactic treatment with an NSAID or colchicine is recommended [see Dosage and Administration (2.4)].
5.3 Hepatic Effects
Cases of fatal and nonfatal hepatic failure in patients taking ULORIC have been reported. During randomized controlled studies, transaminase elevations greater than three times the upper limit of normal (ULN) were observed (AST: 2%, 2%, and ALT: 3%, 2% in ULORIC and allopurinol-treated patients, respectively). No dose-effect relationship for these transaminase elevations was noted [see Clinical Pharmacology (12.3)].
Obtain a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) as a baseline before initiating ULORIC.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient presents abnormal liver tests (ALT or AST greater than three times the upper limit of the reference range), interrupt ULORIC treatment while investigating the probable cause. Permanently discontinue ULORIC if liver injury is confirmed, and no alternate etiology can be found.
Permanently discontinue ULORIC in patients who have serum ALT or AST greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies because they are at risk for severe drug-induced liver injury. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with ULORIC can be used with close monitoring.
5.4 Serious Skin Reactions
Serious skin and hypersensitivity reactions, including Stevens-Johnson Syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS) and toxic epidermal necrolysis (TEN) have been reported postmarketing in patients taking ULORIC. Discontinue ULORIC if serious skin reactions are suspected [see Patient Counseling Information (17)]. Many of these patients had reported previous similar skin reactions to allopurinol. ULORIC should be used with close monitoring in these patients.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the prescribing information:
- Cardiovascular Death [see Warnings and Precautions (5.1)]
- Hepatic Effects [see Warnings and Precautions (5.3)]
- Serious Skin Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Phase 2 and 3 clinical studies, a total of 2757 patients with hyperuricemia and gout were treated with ULORIC 40 mg or 80 mg daily. For ULORIC 40 mg, 559 patients were treated for ≥6 months. For ULORIC 80 mg, 1377 patients were treated for ≥6 months, 674 patients were treated for ≥1 year and 515 patients were treated for ≥2 years. In the CARES study, a total of 3098 patients were treated with ULORIC 40 mg or 80 mg daily; of these, 2155 patients were treated for ≥1 year and 1539 were treated for ≥2 years [see Clinical Studies (14.2)].
Most Common Adverse Reactions
In three randomized, controlled clinical studies (Studies 1, 2 and 3), which were 6 to 12 months in duration, the following adverse reactions were reported by the treating physician as related to study drug. Table 1 summarizes adverse reactions reported at a rate of at least 1% in ULORIC treatment groups and at least 0.5% greater than placebo.
Table 1: Adverse Reactions Occurring in ≥1% of Patients Treated with ULORIC and at Least 0.5% Greater than in Patients Receiving Placebo in Controlled Studies Placebo ULORIC allopurinol* Adverse Reactions (N=134) 40 mg daily
(N=757)80 mg daily
(N=1279)(N=1277) - *
- Of the patients who received allopurinol, 10 received 100 mg, 145 received 200 mg, and 1122 received 300 mg, based on level of renal impairment.
Liver Function Abnormalities 0.7% 6.6% 4.6% 4.2% Nausea 0.7% 1.1% 1.3% 0.8% Arthralgia 0% 1.1% 0.7% 0.7% Rash 0.7% 0.5% 1.6% 1.6% The most common adverse reaction leading to discontinuation from therapy was liver function abnormalities in 1.8% of ULORIC 40 mg, 1.2% of ULORIC 80 mg, and in 0.9% of patients treated with allopurinol.
In addition to the adverse reactions presented in Table 1, dizziness was reported in more than 1% of patients treated with ULORIC although not at a rate more than 0.5% greater than placebo.
In the CARES study, liver function abnormalities and diarrhea were reported in more than 1% of patients treated with ULORIC, although not at a rate more than 0.5% greater than allopurinol.
Less Common Adverse Reactions
In clinical studies the following adverse reactions occurred in less than 1% of patients and in more than one subject treated with doses ranging from 40 mg to 240 mg of ULORIC. This list also includes adverse reactions (less than 1% of patients) associated with organ systems from Warnings and Precautions.
Blood and Lymphatic System Disorders: anemia, idiopathic thrombocytopenic purpura, leukocytosis/leukopenia, neutropenia, pancytopenia, splenomegaly, thrombocytopenia.
Cardiac Disorders: angina pectoris, atrial fibrillation/flutter, cardiac murmur, ECG abnormal, palpitations, sinus bradycardia, tachycardia.
Ear and Labyrinth Disorders: deafness, tinnitus, vertigo.
Eye Disorders: vision blurred.
Gastrointestinal Disorders: abdominal distention, abdominal pain, constipation, dry mouth, dyspepsia, flatulence, frequent stools, gastritis, gastroesophageal reflux disease, gastrointestinal discomfort, gingival pain, hematemesis, hyperchlorhydria, hematochezia, mouth ulceration, pancreatitis, peptic ulcer, vomiting.
General Disorders and Administration Site Conditions: asthenia, chest pain/discomfort, edema, fatigue, feeling abnormal, gait disturbance, influenza-like symptoms, mass, pain, thirst.
Hepatobiliary Disorders: cholelithiasis/cholecystitis, hepatic steatosis, hepatitis, hepatomegaly.
Immune System Disorder: hypersensitivity.
Infections and Infestations: herpes zoster.
Procedural Complications: contusion.
Metabolism and Nutrition Disorders: anorexia, appetite decreased/increased, dehydration, diabetes mellitus, hypercholesterolemia, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypokalemia, weight decreased/increased.
Musculoskeletal and Connective Tissue Disorders: arthritis, joint stiffness, joint swelling, muscle spasms/twitching/tightness/weakness, musculoskeletal pain/stiffness, myalgia.
Nervous System Disorders: altered taste, balance disorder, cerebrovascular accident, Guillain-Barré syndrome, headache, hemiparesis, hypoesthesia, hyposmia, lacunar infarction, lethargy, mental impairment, migraine, paresthesia, somnolence, transient ischemic attack, tremor.
Psychiatric Disorders: agitation, anxiety, depression, insomnia, irritability, libido decreased, nervousness, panic attack, personality change.
Renal and Urinary Disorders: hematuria, nephrolithiasis, pollakiuria, proteinuria, renal failure, renal insufficiency, urgency, incontinence.
Reproductive System and Breast Changes: breast pain, erectile dysfunction, gynecomastia.
Respiratory, Thoracic and Mediastinal Disorders: bronchitis, cough, dyspnea, epistaxis, nasal dryness, paranasal sinus hypersecretion, pharyngeal edema, respiratory tract congestion, sneezing, throat irritation, upper respiratory tract infection.
Skin and Subcutaneous Tissue Disorders: alopecia, angio edema, dermatitis, dermographism, ecchymosis, eczema, hair color changes, hair growth abnormal, hyperhidrosis, peeling skin, petechiae, photosensitivity, pruritus, purpura, skin discoloration/altered pigmentation, skin lesion, skin odor abnormal, urticaria.
Vascular Disorders: flushing, hot flush, hypertension, hypotension.
Laboratory Parameters: activated partial thromboplastin time prolonged, creatine increased, bicarbonate decreased, sodium increased, EEG abnormal, glucose increased, cholesterol increased, triglycerides increased, amylase increased, potassium increased, TSH increased, platelet count decreased, hematocrit decreased, hemoglobin decreased, MCV increased, RBC decreased, creatinine increased, blood urea increased, BUN/creatinine ratio increased, creatine phosphokinase (CPK) increased, alkaline phosphatase increased, LDH increased, PSA increased, urine output increased/decreased, lymphocyte count decreased, neutrophil count decreased, WBC increased/decreased, coagulation test abnormal, low density lipoprotein (LDL) increased, prothrombin time prolonged, urinary casts, urine positive for white blood cells and protein.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ULORIC. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: agranulocytosis, eosinophilia.
Hepatobiliary Disorders: hepatic failure (some fatal), jaundice, serious cases of abnormal liver function test results, liver disorder.
Immune System Disorders: anaphylaxis, anaphylactic reaction.
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis.
Psychiatric Disorders: psychotic behavior including aggressive thoughts.
Renal and Urinary Disorders: tubulointerstitial nephritis.
Skin and Subcutaneous Tissue Disorders: generalized rash, Stevens-Johnson Syndrome, hypersensitivity skin reactions, erythema multiforme, drug reaction with eosinophilia and systemic symptoms, toxic epidermal necrolysis.
-
7 DRUG INTERACTIONS
7.1 Xanthine Oxidase Substrate Drugs
ULORIC is an XO inhibitor. Based on a drug interaction study in healthy patients, febuxostat altered the metabolism of theophylline (a substrate of XO) in humans [see Clinical Pharmacology (12.3)]. Therefore, use with caution when coadministering ULORIC with theophylline.
A drug interaction study of ULORIC and azathioprine, also metabolized by XO, showed an increase in exposure of 6-mercaptopurine which may lead to toxicity [see Clinical Pharmacology (12.3)]. Drug interaction studies of ULORIC with other drugs that are metabolized by XO (e.g., mercaptopurine) have not been conducted. ULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine [see Contraindications (4)].
7.2 Cytotoxic Chemotherapy Drugs
Drug interaction studies of ULORIC with cytotoxic chemotherapy have not been conducted. No data are available regarding the safety of ULORIC during cytotoxic chemotherapy.
7.3 In Vivo Drug Interaction Studies
Based on drug interaction studies in healthy patients, ULORIC does not have clinically significant interactions with colchicine, naproxen, indomethacin, hydrochlorothiazide, warfarin or desipramine [see Clinical Pharmacology (12.3)]. Therefore, ULORIC may be used concomitantly with these medications.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with ULORIC use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. No adverse developmental effects were observed in embryo-fetal development studies with oral administration of febuxostat to pregnant rats and rabbits during organogenesis at doses that produced maternal exposures up to 40 and 51 times, respectively, the exposure at the maximum recommended human dose (MRHD). No adverse developmental effects were observed in a pre- and postnatal development study with administration of febuxostat to pregnant rats from organogenesis through lactation at an exposure approximately 11 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation Days 7 – 17, febuxostat was not teratogenic and did not affect fetal development or survival at exposures up to approximately 40 times the MRHD (on an AUC basis at maternal oral doses up to 48 mg/kg/day). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation Days 6 – 18, febuxostat was not teratogenic and did not affect fetal development at exposures up to approximately 51 times the MRHD (on an AUC basis at maternal oral doses up to 48 mg/kg/day).
In a pre- and postnatal development study in pregnant female rats dosed orally from gestation Day 7 through lactation Day 20, febuxostat had no effects on delivery or growth and development of offspring at a dose approximately 11 times the MRHD (on an AUC basis at a maternal oral dose of 12 mg/kg/day). However, increased neonatal mortality and a reduction in neonatal body weight gain were observed in the presence of maternal toxicity at a dose approximately 40 times the MRHD (on an AUC basis at a maternal oral dose of 48 mg/kg/day).
Febuxostat crossed the placental barrier following oral administration to pregnant rats and was detected in fetal tissues.
8.2 Lactation
Risk Summary
There are no data on the presence of febuxostat in human milk, the effects on the breastfed infant, or the effects on milk production. Febuxostat is present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ULORIC and any potential adverse effects on the breastfed child from ULORIC or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of ULORIC in pediatric patients have not been established.
8.5 Geriatric Use
No dose adjustment is necessary in elderly patients. Of the total number of patients in Studies 1, 2, and 3 (clinical studies of ULORIC in the treatment of gout) [see Clinical Studies (14.1)], 16% were 65 and over, while 4% were 75 and over. Comparing patients in different age groups, no clinically significant differences in safety or effectiveness were observed but greater sensitivity of some older individuals cannot be ruled out. The Cmax and AUC24 of febuxostat following multiple oral doses of ULORIC in geriatric patients (≥65 years) were similar to those in younger patients (18 to 40 years) [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dose adjustment is necessary in patients with mild to moderate renal impairment (Clcr 30 to 89 mL/min). For patients with severe renal impairment (Clcr 15 to 29 mL/min), the recommended dosage of ULORIC is limited to 40 mg once daily [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh Class A or B). No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C); therefore, caution should be exercised in these patients [see Clinical Pharmacology (12.3)].
8.8 Secondary Hyperuricemia
No studies have been conducted in patients with secondary hyperuricemia (including organ transplant recipients); ULORIC is not recommended for use in patients whom the rate of urate formation is greatly increased (e.g., malignant disease and its treatment, Lesch-Nyhan syndrome). The concentration of xanthine in urine could, in rare cases, rise sufficiently to allow deposition in the urinary tract.
- 10 OVERDOSAGE
-
11 DESCRIPTION
ULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The empirical formula is C16H16N2O3S.
The chemical structure is:
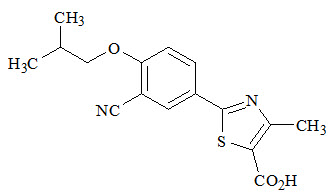
Febuxostat is a non-hygroscopic, white crystalline powder that is freely soluble in dimethylformamide; soluble in dimethylsulfoxide; sparingly soluble in ethanol; slightly soluble in methanol and acetonitrile; and practically insoluble in water. The melting range is 205°C to 208°C.
ULORIC tablets for oral use contain the active ingredient, febuxostat, and are available in two dosage strengths, 40 mg and 80 mg. Inactive ingredients include hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium croscarmellose. ULORIC tablets are coated with Opadry II, green.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ULORIC, a xanthine oxidase inhibitor, achieves its therapeutic effect by decreasing serum uric acid. ULORIC is not expected to inhibit other enzymes involved in purine and pyrimidine synthesis and metabolism at therapeutic concentrations.
12.2 Pharmacodynamics
Effect on Uric Acid and Xanthine Concentrations
In healthy patients, ULORIC resulted in a dose dependent decrease in 24-hour mean serum uric acid concentrations and an increase in 24-hour mean serum xanthine concentrations. In addition, there was a decrease in the total daily urinary uric acid excretion. Also, there was an increase in total daily urinary xanthine excretion. Percent reduction in 24-hour mean serum uric acid concentrations was between 40% and 55% at the exposure levels of 40 mg and 80 mg daily doses.
Effect on Cardiac Repolarization
The effect of ULORIC on cardiac repolarization as assessed by the QTc interval was evaluated in normal healthy patients and in patients with gout. ULORIC in doses up to 300 mg daily (3.75 times the maximum recommended daily dosage), at steady-state, did not demonstrate an effect on the QTc interval.
12.3 Pharmacokinetics
In healthy patients, maximum plasma concentrations (Cmax) and AUC of febuxostat increased in a dose proportional manner following single and multiple doses of 10 mg (0.25 times the lowest recommended dosage) to 120 mg (1.5 times the maximum recommended dosage). There is no accumulation when therapeutic doses are administered every 24 hours. Febuxostat has an apparent mean terminal elimination half-life (t1/2) of approximately 5 to 8 hours. Febuxostat pharmacokinetic parameters for patients with hyperuricemia and gout estimated by population pharmacokinetic analyses were similar to those estimated in healthy patients.
Absorption
The absorption of radiolabeled febuxostat following oral dose administration was estimated to be at least 49% (based on total radioactivity recovered in urine). Maximum plasma concentrations of febuxostat occurred between 1 and 1.5 hours postdose. After multiple oral 40 mg and 80 mg once daily doses, Cmax is approximately 1.6 ± 0.6 mcg/mL (N=30), and 2.6 ± 1.7 mcg/mL (N=227), respectively. Absolute bioavailability of the febuxostat tablet has not been studied.
Following multiple 80 mg once daily doses with a high fat meal, there was a 49% decrease in Cmax and an 18% decrease in AUC, respectively. However, no clinically significant change in the percent decrease in serum uric acid concentration was observed (58% fed vs 51% fasting). Thus, ULORIC may be taken without regard to food.
Concomitant ingestion of an antacid containing magnesium hydroxide and aluminum hydroxide with an 80 mg single dose of ULORIC has been shown to delay absorption of febuxostat (approximately one hour) and to cause a 31% decrease in Cmax and a 15% decrease in AUC∞. As AUC rather than Cmax was related to drug effect, change observed in AUC was not considered clinically significant. Therefore, ULORIC may be taken without regard to antacid use.
Distribution
The mean apparent steady state volume of distribution (Vss/F) of febuxostat was approximately 50 L (CV ~40%). The plasma protein binding of febuxostat is approximately 99.2% (primarily to albumin), and is constant over the concentration range achieved with 40 mg and 80 mg doses.
Metabolism
Febuxostat is extensively metabolized by both conjugation via uridine diphosphate glucuronosyltransferase (UGT) enzymes including UGT1A1, UGT1A3, UGT1A9, and UGT2B7 and oxidation via cytochrome P450 (CYP) enzymes including CYP1A2, 2C8 and 2C9 and non-P450 enzymes. The relative contribution of each enzyme isoform in the metabolism of febuxostat is not clear. The oxidation of the isobutyl side chain leads to the formation of four pharmacologically active hydroxy metabolites, all of which occur in plasma of humans at a much lower extent than febuxostat.
In urine and feces, acyl glucuronide metabolites of febuxostat (~35% of the dose), and oxidative metabolites, 67M-1 (~10% of the dose), 67M-2 (~11% of the dose), and 67M-4, a secondary metabolite from 67M-1 (~14% of the dose), appeared to be the major metabolites of febuxostat in vivo.
Elimination
Febuxostat is eliminated by both hepatic and renal pathways. Following an 80 mg oral dose of 14C-labeled febuxostat, approximately 49% of the dose was recovered in the urine as unchanged febuxostat (3%), the acyl glucuronide of the drug (30%), its known oxidative metabolites and their conjugates (13%), and other unknown metabolites (3%). In addition to the urinary excretion, approximately 45% of the dose was recovered in the feces as the unchanged febuxostat (12%), the acyl glucuronide of the drug (1%), its known oxidative metabolites and their conjugates (25%), and other unknown metabolites (7%).
The apparent mean terminal elimination half-life (t1/2) of febuxostat was approximately 5 to 8 hours.
Specific Populations
Geriatric Patients
The Cmax and AUC of febuxostat and its metabolites following multiple oral doses of ULORIC in geriatric patients (≥65 years) were similar to those in younger patients (18 to 40 years). In addition, the percent decrease in serum uric acid concentration was similar between elderly and younger patients. No dose adjustment is necessary in geriatric patients [see Use in Specific Populations (8.5)].
Patients with Renal Impairment
In a dedicated phase I pharmacokinetics study, following multiple 80 mg doses of ULORIC in healthy patients with mild (Clcr 50 to 80 mL/min), moderate (Clcr 30 to 49 mL/min) or severe renal impairment (Clcr 10 to 29 mL/min), the Cmax of febuxostat did not change relative to patients with normal renal function (Clcr greater than 80 mL/min). AUC and half-life of febuxostat increased in patients with renal impairment in comparison to patients with normal renal function, but values were similar among three renal impairment groups. Mean febuxostat AUC values were up to 1.8 times higher in patients with renal impairment compared to those with normal renal function. Mean Cmax and AUC values for three active metabolites increased up to two and four-fold, respectively. However, the percent decrease in serum uric acid concentration for patients with renal impairment was comparable to those with normal renal function (58% in normal renal function group and 55% in the severe renal function group).
Based on population pharmacokinetic analysis, following multiple 40 mg or 80 mg doses of ULORIC, the mean oral clearance (CL/F) values of febuxostat in patients with gout and mild (n=334), moderate (n=232) or severe (n=34) renal impairment were decreased by 14%, 34%, and 48%, respectively, compared to patients with normal (n=89) renal function. The corresponding median AUC values of febuxostat at steady-state in patients with renal impairment were increased by 18%, 49%, and 96% after 40 mg dose, and 7%, 45% and 98% after 80 mg dose, respectively, compared to patients with normal renal function.
ULORIC has not been studied in end stage renal impairment patients who are on dialysis.
Patients with Hepatic Impairment
Following multiple 80 mg doses of ULORIC in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment, an average of 20% to 30% increase was observed for both Cmax and AUC24 (total and unbound) in hepatic impairment groups compared to patients with normal hepatic function. In addition, the percent decrease in serum uric acid concentration was comparable between different hepatic groups (62% in healthy group, 49% in mild hepatic impairment group, and 48% in moderate hepatic impairment group). No dose adjustment is necessary in patients with mild or moderate hepatic impairment. No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C); caution should be exercised in those patients [see Use in Specific Populations (8.7)].
Male and Female Patients
Following multiple oral doses of ULORIC, the Cmax and AUC24 of febuxostat were 30% and 14% higher in females than in males, respectively. However, weight-corrected Cmax and AUC were similar between the genders. In addition, the percent decrease in serum uric acid concentrations was similar between genders. No dose adjustment is necessary based on gender.
Drug Interaction Studies
Effect of ULORIC on Other Drugs
Xanthine Oxidase Substrate Drugs-Azathioprine, Mercaptopurine, and Theophylline
Febuxostat is an XO inhibitor. A drug-drug interaction study evaluating the effect of ULORIC upon the pharmacokinetics of theophylline (an XO substrate) in healthy patients showed that coadministration of febuxostat with theophylline resulted in an approximately 400-fold increase in the amount of 1-methylxanthine, one of the major metabolites of theophylline, excreted in the urine. Since the long-term safety of exposure to 1-methylxanthine in humans is unknown, use with caution when coadministering febuxostat with theophylline.
A drug interaction study of ULORIC and azathioprine has been conducted [see Drug Interactions (7)]. Inhibition of XO by ULORIC caused increased plasma concentrations of 6-mercaptopurine, a metabolite of azathioprine, which may lead to toxicity. Drug interaction studies of ULORIC with other drugs that are metabolized by XO (e.g., mercaptopurine) have not been conducted. ULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine [see Contraindications (4) and Drug Interactions (7)].
Azathioprine and mercaptopurine undergo metabolism via three major metabolic pathways, one of which is mediated by XO. Concomitant administration of allopurinol [a xanthine oxidase inhibitor] with azathioprine or mercaptopurine has been reported to substantially increase plasma concentrations of these drugs. Because ULORIC is a xanthine oxidase inhibitor, it could inhibit the XO-mediated metabolism of mercaptopurine leading to increased plasma concentrations of mercaptopurine that could result in severe toxicity.
P450 Substrate Drugs
In vitro studies have shown that febuxostat does not inhibit P450 enzymes CYP1A2, 2C9, 2C19, 2D6, or 3A4 and it also does not induce CYP1A2, 2B6, 2C9, 2C19, or 3A4 at clinically relevant concentrations. As such, pharmacokinetic interactions between ULORIC and drugs metabolized by these CYP enzymes are unlikely.
Effect of Other Drugs on ULORIC
Febuxostat is metabolized by conjugation and oxidation via multiple metabolizing enzymes. The relative contribution of each enzyme isoform is not clear. Drug interactions between ULORIC and a drug that inhibits or induces one particular enzyme isoform is in general not expected.
In Vivo Drug Interaction Studies
Azathioprine
Concomitant use of ULORIC and azathioprine is contraindicated. Coadministration with febuxostat (40 mg or 120 mg QD) reduced the apparent clearance of mercaptopurine, a XO substrate, by 83.2% to 83.8% following a single oral dose of azathioprine, a prodrug of 6-mercaptopurine. No significant differences were observed in the extent of inhibition of 6-mercaptopurine metabolism by febuxostat 40 mg and 120 mg.
Theophylline
No dose adjustment is necessary for theophylline when coadministered with ULORIC. Administration of ULORIC (80 mg once daily) with theophylline resulted in an increase of 6% in Cmax and 6.5% in AUC of theophylline. These changes were not considered statistically significant. However, the study also showed an approximately 400-fold increase in the amount of 1-methylxanthine (one of the major theophylline metabolites) excreted in urine as a result of XO inhibition by ULORIC. The safety of long-term exposure to 1-methylxanthine has not been evaluated. This should be taken into consideration when deciding to coadminister ULORIC and theophylline.
Colchicine
No dose adjustment is necessary for either ULORIC or colchicine when the two drugs are coadministered. Administration of ULORIC (40 mg once daily) with colchicine (0.6 mg twice daily) resulted in an increase of 12% in Cmax and 7% in AUC24 of febuxostat. In addition, administration of colchicine (0.6 mg twice daily) with ULORIC (120 mg daily) resulted in a less than 11% change in Cmax or AUC of colchicine for both AM and PM doses. These changes were not considered clinically significant.
Naproxen
No dose adjustment is necessary for ULORIC or naproxen when the two drugs are coadministered. Administration of ULORIC (80 mg once daily) with naproxen (500 mg twice daily) resulted in a 28% increase in Cmax and a 40% increase in AUC of febuxostat. The increases were not considered clinically significant. In addition, there were no significant changes in the Cmax or AUC of naproxen (less than 2%).
Indomethacin
No dose adjustment is necessary for either ULORIC or indomethacin when these two drugs are coadministered. Administration of ULORIC (80 mg once daily) with indomethacin (50 mg twice daily) did not result in any significant changes in Cmax or AUC of febuxostat or indomethacin (less than 7%).
Hydrochlorothiazide
No dose adjustment is necessary for ULORIC when coadministered with hydrochlorothiazide. Administration of ULORIC (80 mg) with hydrochlorothiazide (50 mg) did not result in any clinically significant changes in Cmax or AUC of febuxostat (less than 4%), and serum uric acid concentrations were not substantially affected.
Warfarin
No dose adjustment is necessary for warfarin when coadministered with ULORIC. Administration of ULORIC (80 mg once daily) with warfarin had no effect on the pharmacokinetics of warfarin in healthy patients. INR and Factor VII activity were also not affected by the coadministration of ULORIC.
Desipramine
Coadministration of drugs that are CYP2D6 substrates (such as desipramine) with ULORIC are not expected to require dose adjustment. Febuxostat was shown to be a weak inhibitor of CYP2D6 in vitro and in vivo. Administration of ULORIC (120 mg once daily) with desipramine (25 mg) resulted in an increase in Cmax (16%) and AUC (22%) of desipramine, which was associated with a 17% decrease in the 2-hydroxydesipramine to desipramine metabolic ratio (based on AUC).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in F344 rats and B6C3F1 mice. Increased transitional cell papilloma and carcinoma of the urinary bladder was observed at 24 mg/kg (25 times the MRHD on an AUC basis and 18.75 mg/kg (12.5 times the MRHD on an AUC basis) in male rats and female mice, respectively. The urinary bladder neoplasms were secondary to calculus formation in the kidney and urinary bladder.
Febuxostat showed a positive clastogenic response in a chromosomal aberration assay in a Chinese hamster lung fibroblast cell line with and without metabolic activation in vitro. Febuxostat was negative in the following genotoxicity assays: the in vitro Ames assay, in vitro chromosomal aberration assay in human peripheral lymphocytes, the L5178Y mouse lymphoma cell line assay, the in vivo mouse micronucleus assay, and the rat unscheduled DNA synthesis assay.
Fertility and reproductive performance were unaffected in male or female rats that received febuxostat at oral doses up to 48 mg/kg/day (approximately 31 and 40 times the MRHD on an AUC basis in males and females respectively).
13.2 Animal Toxicology
A 12-month toxicity study in beagle dogs showed deposition of xanthine crystals and calculi in kidneys at 15 mg/kg (approximately 4 times the MRHD on an AUC basis). A similar effect of calculus formation was noted in rats in a 6-month study due to deposition of xanthine crystals at 48 mg/kg (approximately 31 and 40 times the MRHD on an AUC basis in males and females respectively).
-
14 CLINICAL STUDIES
A serum uric acid level of less than 6 mg/dL is the goal of antihyperuricemic therapy and has been established as appropriate for the treatment of gout.
14.1 Management of Hyperuricemia in Gout
The efficacy of ULORIC was demonstrated in three randomized, double-blind, controlled trials in patients with hyperuricemia and gout. Hyperuricemia was defined as a baseline serum uric acid level ≥8 mg/dL.
Study 1 (NCT00430248) randomized patients to: ULORIC 40 mg daily, ULORIC 80 mg daily, or allopurinol (300 mg daily for patients with estimated creatinine clearance (Clcr) ≥60 mL/min or 200 mg daily for patients with estimated Clcr ≥30 mL/min and ≤59 mL/min). The duration of Study 1 was six months.
Study 2 (NCT00174915) randomized patients to: placebo, ULORIC 80 mg daily, ULORIC 120 mg daily, ULORIC 240 mg daily or allopurinol (300 mg daily for patients with a baseline serum creatinine ≤1.5 mg/dL or 100 mg daily for patients with a baseline serum creatinine greater than 1.5 mg/dL and ≤2 mg/dL). The duration of Study 2 was six months.
Study 3 (NCT00102440), a year study, randomized patients to: ULORIC 80 mg daily, ULORIC 120 mg daily, or allopurinol 300 mg daily. Patients who completed Study 2 and Study 3 were eligible to enroll in a Phase 3 long-term extension study in which patients received treatment with ULORIC for over three years.
In all three studies, patients received naproxen 250 mg twice daily or colchicine 0.6 mg once or twice daily for gout flare prophylaxis. In Study 1 the duration of prophylaxis was six months; in Study 2 and Study 3 the duration of prophylaxis was eight weeks.
The efficacy of ULORIC was also evaluated in a 4-week dose ranging study which randomized patients to: placebo, ULORIC 40 mg daily, ULORIC 80 mg daily, or ULORIC 120 mg daily. Patients who completed this study were eligible to enroll in a long-term extension study in which patients received treatment with ULORIC for up to five years.
Patients in these studies were representative of the patient population for which ULORIC use is intended. Table 2 summarizes the demographics and baseline characteristics for the patients enrolled in the studies.
Table 2: Patient Demographics and Baseline Characteristics in Study 1, Study 2, and Study 3 Male 95% Race: Caucasian 80% African American 10% Ethnicity: Hispanic or Latino 7% Alcohol User 67% Mild to Moderate Renal Insufficiency
(percent with estimated Clcr less than 90 mL/min)59% History of Hypertension 49% History of Hyperlipidemia 38% BMI ≥30 kg/m2 63% Mean BMI 33 kg/m2 Baseline sUA ≥10 mg/dL 36% Mean baseline sUA 9.7 mg/dL Experienced a gout flare in previous year 85% Serum Uric Acid Level less than 6 mg/dL at Final Visit
ULORIC 80 mg was superior to allopurinol in lowering serum uric acid to less than 6 mg/dL at the final visit. ULORIC 40 mg daily, although not superior to allopurinol, was effective in lowering serum uric acid to less than 6 mg/dL at the final visit (Table 3).
Table 3: Proportion of Patients with Serum Uric Acid Levels less than 6 mg/dL at Final Visit Difference in Proportion
(95% CI)Study* ULORIC
40 mg dailyULORIC
80 mg dailyallopurinol Placebo ULORIC
40 mg
vs
allopurinolULORIC
80 mg
vs
allopurinol- *
- Randomization was balanced between treatment groups, except in Study 2 in which twice as many patients were randomized to each of the active treatment groups compared to placebo.
Study 1
(6 months)
(N=2268)45% 67% 42% 3%
(-2%, 8%)25%
(20%, 30%)Study 2
(6 months)
(N=643)72% 39% 1% 33%
(26%, 42%)Study 3
(12 months)
(N=491)74% 36% 38%
(30%, 46%)In 76% of ULORIC 80 mg patients, reduction in serum uric acid levels to less than 6 mg/dL was noted by the Week 2 visit. Average serum uric acid levels were maintained at 6 mg/dL or below throughout treatment in 83% of these patients.
In all treatment groups, fewer patients with higher baseline serum urate levels (≥10 mg/dL) and/or tophi achieved the goal of lowering serum uric acid to less than 6 mg/dL at the final visit; however, a higher proportion achieved a serum uric acid less than 6 mg/dL with ULORIC 80 mg than with ULORIC 40 mg or allopurinol.
Study 1 evaluated efficacy in patients with mild to moderate renal impairment (i.e., baseline estimated Clcr less than 90 mL/min). The results in this subgroup of patients are shown in Table 4.
Table 4: Proportion of Patients with Serum Uric Acid Levels less than 6 mg/dL in Patients with Mild or Moderate Renal Impairment at Final Visit Difference in Proportion
(95% CI)ULORIC
40 mg daily
(N=479)ULORIC
80 mg daily
(N=503)allopurinol*
300 mg daily
(N=501)ULORIC
40 mg
vs
allopurinolULORIC
80 mg
vs
allopurinol- *
- Allopurinol patients (n=145) with estimated Clcr ≥30 mL/min and Clcr ≤59 mL/min were dosed at 200 mg daily.
50% 72% 42% 7%
(1%, 14%)29%
(23%, 35%)14.2 Cardiovascular Safety Study
A randomized, double-blind, allopurinol-controlled CV outcomes study (CARES) was conducted to evaluate the CV risk of ULORIC (NCT01101035). The study compared the risk of MACE between patients treated with ULORIC (N=3098) and allopurinol-treated patients (N=3092). The primary endpoint was the time to first occurrence of a MACE defined as the composite of CV death, nonfatal MI, nonfatal stroke, or unstable angina with urgent coronary revascularization. The study was designed to exclude a prespecified risk margin of 1.3 for the hazard ratio of MACE. An independent committee conducted a blinded evaluation of serious CV adverse events according to predefined criteria (adjudication) for determination of MACE. The study was event driven and patients were followed until a sufficient number of primary outcome events accrued. The median on-study follow-up time was 2.6 years.
Patients randomized to ULORIC initially received 40 mg once daily which was increased to 80 mg once daily, if their sUA was ≥6mg/dL at Week 2. For patients randomized to allopurinol, those who had normal renal function or mild renal impairment (estimated creatinine clearance (eClcr) ≥60 to ˂90 mL/minute) initially received 300 mg once daily with 100 mg/day dose increments monthly until either sUA ˂6mg/dL or an allopurinol dosage of 600 mg once daily was achieved; those who had moderate renal impairment (eClcr ≥30 to ˂60 mL/minute) initially received 200 mg once daily with 100 mg/day dose increments monthly until either a sUA ˂6 mg/dL or an allopurinol dosage of 400 mg once daily was achieved.
The mean age of the population was 65 years (range: 44 to 93 years). Most patients were male (84%) and Caucasian (69%). Patients had a diagnosis of gout for approximately 12 years, a mean baseline sUA of 8.7 mg/dL, and 90% had experienced at least one gout flare in the past year. CV history included MI (39%), hospitalization for unstable angina (28%), cardiac revascularization (37%), and stroke (14%). The most prevalent comorbid conditions were hypertension (92%), hyperlipidemia (87%), diabetes mellitus (55%), diabetes mellitus with micro- or macrovascular disease (39%), and renal impairment [92% with an eClcr 30 to 89 mL/minute]. The use of CV disease medication was balanced across treatment groups. Baseline CV disease medications included: ACE inhibitors or ARBs (70%), lipid modifying agents (74%), aspirin (62%), beta-blockers (59%), calcium channel blockers (26%), and nonaspirin antiplatelet medications (31%).
Table 5 shows the study results for the primary MACE composite endpoint and its individual components. For the composite primary endpoint, the ULORIC group was non-inferior compared with the allopurinol group. The rates of nonfatal MI, stroke, and unstable angina with urgent coronary revascularization were similar. There was a higher rate of CV deaths in patients treated with ULORIC (134 CV deaths; 1.5 per 100 PY) than in allopurinol-treated patients (100 CV deaths; 1.1 per 100 PY). Sudden cardiac death was the most common cause of adjudicated CV deaths in the ULORIC group (83 of 3,098; 2.7%) as compared to the allopurinol group (56 of 3,092; 1.8%). The biological plausibility of CV death associated with ULORIC is unclear.
All-cause mortality was higher in the ULORIC group (243 deaths [7.8%]; 2.6 per 100 PY) than the allopurinol group (199 deaths [6.4%]; 2.2 per 100 PY) [Hazard Ratio: 1.22, 95% CI: 1.01, 1.47], due to a higher rate of CV deaths.
Table 5: Patients with MACE in CARES (Cardiovascular Outcomes Study in Patients with Gout) ULORIC
N=3098Allopurinol
N=3092Hazard Ratio Number of Patients with Event (%) Rate per 100 PY* Number of Patients with Event (%) Rate per 100 PY* 95% CI - *
- Patient Years (PY)
Composite of primary endpoint MACE 335 (10.8) 3.8 321 (10.4) 3.7 1.03 (0.89, 1.21) Cardiovascular Death 134 (4.3) 1.5 100 (3.2) 1.1 1.34 (1.03, 1.73) Nonfatal MI 111 (3.6) 1.2 118 (3.8) 1.3 0.93 (0.72, 1.21) Nonfatal stroke 71 (2.3) 0.8 70 (2.3) 0.8 1.01 (0.73, 1.41) Unstable angina with urgent coronary revascularization 49 (1.6) 0.5 56 (1.8) 0.6 0.86 (0.59, 1.26) -
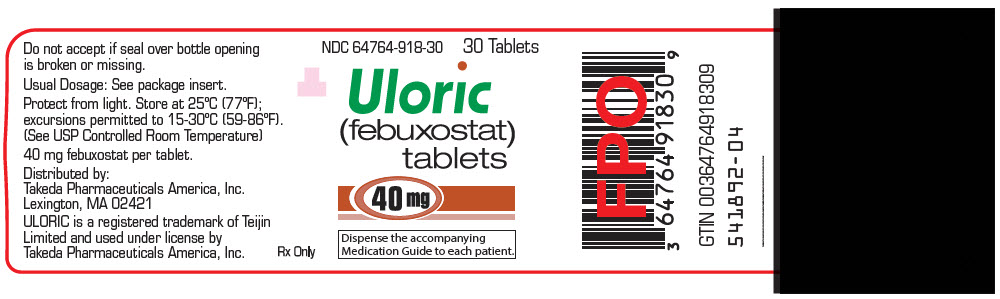
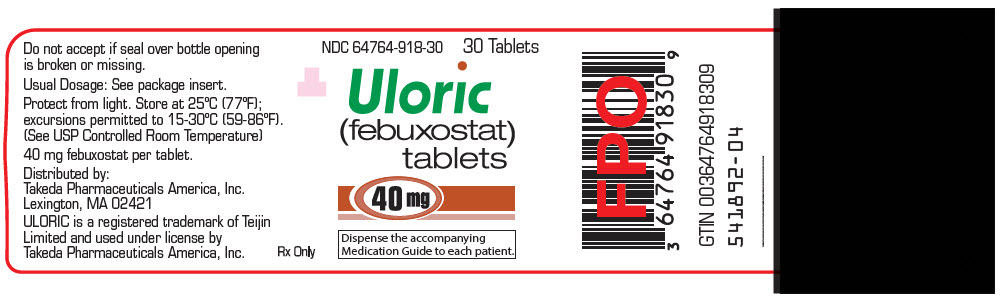
16 HOW SUPPLIED/STORAGE AND HANDLING
ULORIC 40 mg tablets are light green to green in color, round, debossed with "TAP" on one side and "40" on the other side and supplied as:
NDC Number Size 64764-918-11 Hospital Unit Dose Pack of 100 Tablets 64764-918-30 Bottle of 30 Tablets 64764-918-90 Bottle of 90 Tablets 64764-918-18 Bottle of 500 Tablets ULORIC 80 mg tablets are light green to green in color, teardrop shaped, debossed with "TAP" on one side and "80" on the other side and supplied as:
NDC Number Size 64764-677-11 Hospital Unit Dose Pack of 100 Tablets 64764-677-30 Bottle of 30 Tablets 64764-677-13 Bottle of 100 Tablets 64764-677-19 Bottle of 1000 Tablets -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
CV Death
Inform patients that gout patients with established CV disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study. Inform all patients of the higher rate of CV death with ULORIC compared to allopurinol. Alert all patients (those with and without CV disease) for the development of signs and symptoms of CV events and to seek medical care promptly should they occur [see Warnings and Precautions (5.1)].
Gout Flares
Inform patients that after initiation of ULORIC an increase in gout flares can occur and that is not reason to stop taking the medication. Instruct patients that it is recommended to initiate and continue gout prophylaxis therapy for six months while taking ULORIC [see Warnings and Precautions (5.2)].
Hepatic Effects
Inform patients that hepatic effects, including fatal ones, have occurred in patients treated with ULORIC. Instruct them to inform their healthcare provider if they experience liver injury symptoms [see Warnings and Precautions (5.3)].
Serious Skin Reactions
Inform patients that serious skin and hypersensitivity reactions have occurred in patients treated with ULORIC. Instruct patients to discontinue ULORIC if they develop symptoms of these reactions [see Warnings and Precautions (5.4)].
-
PATIENT PACKAGE INSERT
This Medication Guide has been approved by the U.S. Food and Drug Administration ULR015 R10 Revised: April 2023 MEDICATION GUIDE
ULORIC (Ū–'lor–ik)
(febuxostat)
tablets, for oral useRead the Medication Guide that comes with ULORIC before you start taking it and each time you get a refill. There may be new information. The Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. What is the most important information that I should know about ULORIC?
ULORIC may cause serious side effects, including:
Heart-related deaths.
Call your doctor or get emergency medical help right away if you have any of the following symptoms, especially if they are new, worse, or worry you:- chest pain
- shortness of breath or trouble breathing
- dizziness, fainting or feeling lightheaded
- rapid or irregular heartbeat
- numbness or weakness on one side of your body
- slurring of speech
- sudden blurry vision or sudden severe headache
What is ULORIC?
ULORIC is a prescription medicine called a xanthine oxidase (XO) inhibitor used to lower blood uric acid levels in adult patients with gout when allopurinol has not worked well enough or when allopurinol is not right for you.
ULORIC is not for use in people who do not have symptoms of high blood uric acid levels.
It is not known if ULORIC is safe and effective in children.Who should not take ULORIC?
Do not take ULORIC if you:- take azathioprine (Azasan, Imuran)
- take mercaptopurine (Purinethol, Purixan)
What should I tell my doctor before taking ULORIC?
Before taking ULORIC tell your doctor about all of your medical conditions, including if you:- have taken allopurinol and what happened to you while you were taking it.
- have a history of heart disease or stroke.
- have liver or kidney problems.
- are pregnant or plan to become pregnant. It is not known if ULORIC will harm your unborn baby. Talk with your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if ULORIC passes into your breast milk. You and your doctor should decide if you should take ULORIC while breastfeeding.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.How should I take ULORIC? - Take ULORIC exactly as your doctor tells you to take it.
- ULORIC can be taken with or without food.
- ULORIC can be taken with antacids.
- Your gout may get worse (flare) when you start taking ULORIC. Do not stop taking ULORIC because you have a flare.
What are the possible side effects of ULORIC?
ULORIC may cause serious side effects, including:- Heart problems. See "What is the most important information I should know about ULORIC?".
- Gout Flares. Gout flares can happen when you start taking ULORIC. Your doctor may give you other medicines to help prevent your gout flares.
- Liver problems. Liver problems can happen in people who take ULORIC. Your doctor may do blood tests to check how well your liver is working before and during your treatment with ULORIC. Tell your doctor if you get any of the following signs or symptoms of liver problems:
- fatigue
- loss of appetite for several days or longer
- pain, aching, or tenderness on the right side of your stomach-area
- dark or "tea-colored" urine
- your skin or the white part of your eyes turns yellow (jaundice)
- Severe skin and allergic reactions. Serious skin and allergic reactions that may affect different parts of the body such as your liver, kidneys, heart or lungs, can happen in people who take ULORIC. Call your doctor right away or get emergency medical help if you have any of the following symptoms:
- rash
- red and painful skin
- severe skin blisters
- peeling skin
- sores around the lips, eyes or mouth
- swollen face, lips, mouth, tongue or throat
- flu-like symptoms
The most common side effects of ULORIC include: - abnormal liver function tests
- nausea
- joint pain
- rash
These are not all of the possible side effects of ULORIC.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ULORIC? - Store ULORIC at room temperature.
- Keep ULORIC out of the light.
General information about the safe and effective use of ULORIC.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ULORIC for a condition for which it was not prescribed. Do not give ULORIC to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about ULORIC that is written for health professionals.What are the ingredients in ULORIC?
Active ingredient: febuxostat
Inactive ingredients: lactose monohydrate, microcrystalline cellulose, hydroxypropyl cellulose, sodium croscarmellose, silicon dioxide, magnesium stearate, and Opadry II, green
Distributed by:
Takeda Pharmaceuticals America, Inc.
Lexington, MA 02421
ULORIC is a registered trademark of Teijin Limited registered in the U.S. Patent and Trademark Office and used under license by Takeda Pharmaceuticals America, Inc.
All other trademarks are the property of their respective owners.
©2009-2023 Takeda Pharmaceuticals America, Inc.
For more information, go to www.ULORIC.com or call 1-877-TAKEDA (1-877-825-3327).541887-04
- PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ULORIC
febuxostat tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64764-918 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength febuxostat (UNII: 101V0R1N2E) (febuxostat - UNII:101V0R1N2E) febuxostat 40 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) croscarmellose sodium (UNII: M28OL1HH48) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Talc (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Titanium Dioxide (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN (light green to green) Score no score Shape ROUND (round shaped) Size 9mm Flavor Imprint Code TAP;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-918-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 2 NDC:64764-918-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 3 NDC:64764-918-18 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 4 NDC:64764-918-11 10 in 1 CARTON 02/13/2009 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021856 02/13/2009 ULORIC
febuxostat tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64764-677 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength febuxostat (UNII: 101V0R1N2E) (febuxostat - UNII:101V0R1N2E) febuxostat 80 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) croscarmellose sodium (UNII: M28OL1HH48) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Talc (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Titanium Dioxide (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN (light green to green) Score no score Shape TEAR (teardrop shaped) Size 14mm Flavor Imprint Code TAP;80 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-677-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 2 NDC:64764-677-13 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 3 NDC:64764-677-19 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/13/2009 4 NDC:64764-677-11 10 in 1 CARTON 02/13/2009 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021856 02/13/2009 Labeler - Takeda Pharmaceuticals America, Inc. (039997266)