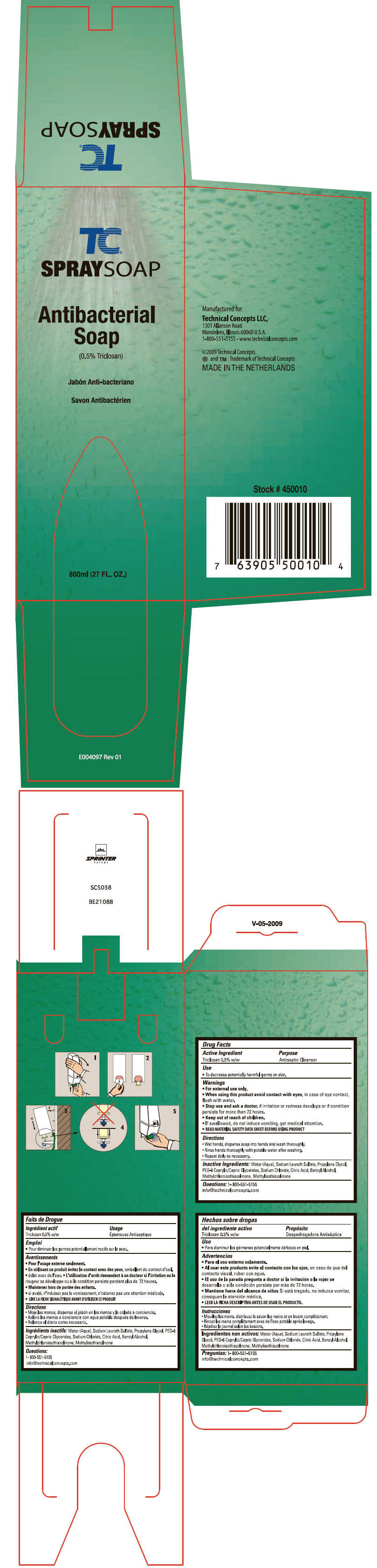
TC SPRAYSOAP- triclosan liquid
Rubbermaid Commercial Products LLC
----------
TC®
SPRAY SOAP
Warnings
- For external use only.
Directions
- Wet hands, dispense soap into hands and wash thoroughly.
- Rinse hands thoroughly with potable water after washing.
- Repeat daily as necessary.
| TC SPRAYSOAP
triclosan liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rubbermaid Commercial Products LLC (049924368) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NWL Netherlands Production B.V. | 489421698 | ANALYSIS(65321-017) , MANUFACTURE(65321-017) | |
Revised: 10/2023
Document Id: f3837c5b-0cfb-4a6f-96e3-35052e07043f
Set id: 532eb1b6-27ac-4cd9-8499-aaff70a25810
Version: 5
Effective Time: 20231030
Rubbermaid Commercial Products LLC