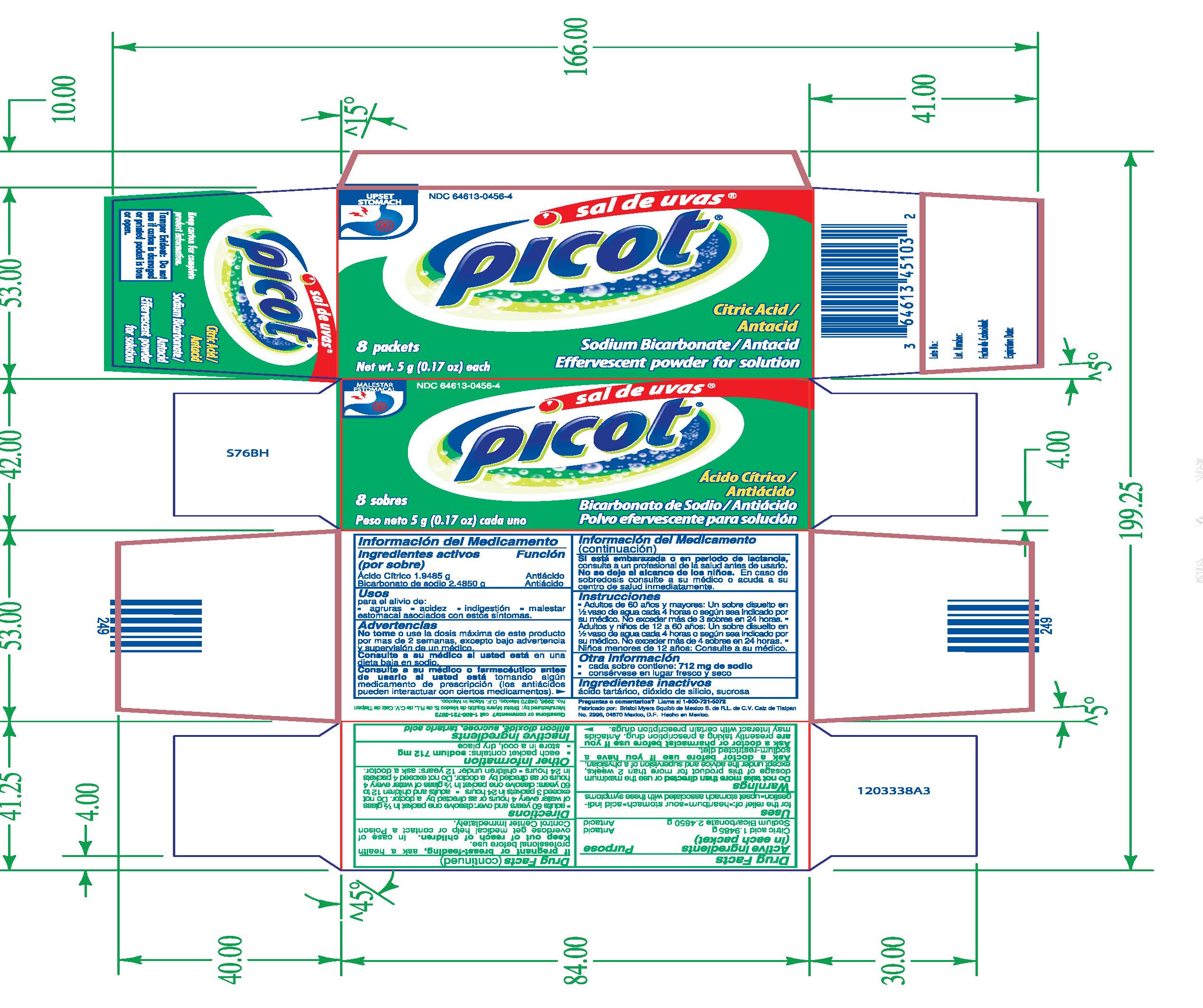
SAL DE UVAS PICOT- citrict acid, sodium bicarbonate powder, for solution
Bristol-Myers Squibb de Mexico, S. de R.L. de C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sal de Uvas Picot
Antacid
Uses
for the relief of:
- Heartburn
- Sour stomach
- acid indigestion
- upset stomach associated with these symptoms
Warnings
Do not take more than directed or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician.
Ask a doctor before use if you have a sodium-restricted diet.
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN
In case of overdose get medical help or contact a Poison Control Center immediately.
Directions
- adults 60 years and over: dissolve one packet in 1/2 glass of water every 4 hours or as directed by a doctor. Do not exceed 3 packets in 24 hours
- adults and children 12 to 60 years: dissolve one packet in 1/2 glass of water every 4 hours or as directed by a doctor. Do not exceed 4 packets in 24 hours.
- children under 12 years: ask a doctor.
sal de uvas picot
Citric Acid/Antacid
Sodium Bicarbonate/Antacid
Effervescent powder for solution
8 packets
Net wt. 5 g (0.17 oz) each
Keep carton for complete product information.
Tamper Evident: Do not use if carton is damaged or printed packet is torn or open.
Questions or Comments? call 1-800-721-5072
Manufactured by: Bristol Myers Squibb de Mexico S. de R.L. de C.V. calz de Tialpan No. 2996, 04870 Mexico
Made in Mexico
Lot No.:
Expiration Date:
8 packets_1203338A3 9 jul 2015.jpg
| SAL DE UVAS PICOT
citrict acid, sodium bicarbonate powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bristol-Myers Squibb de Mexico, S. de R.L. de C.V. (823646211) |