Label: TOTAL WHITE BRIGHTENING- hydroquinone, ethylhexyl methoxycinnamate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 53218-002-02, 53218-002-03, 53218-002-04 - Packager: YZY Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 7, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
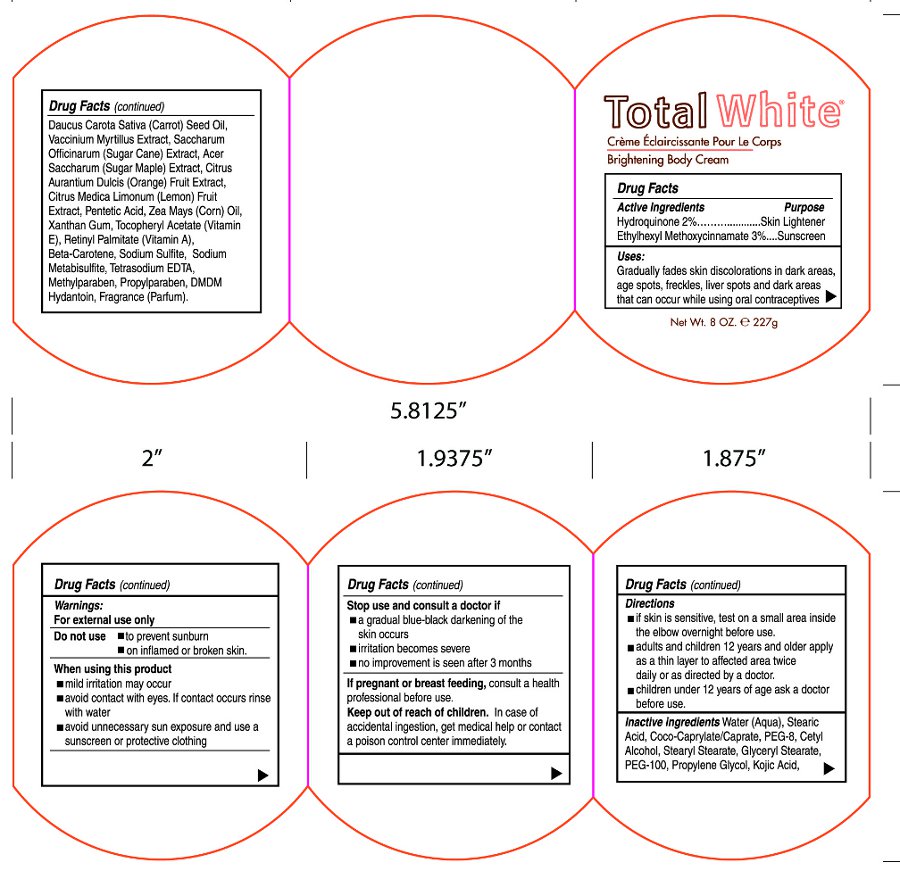
- Active Ingredients
- Purpose
- Uses:
- Warnings:
- Do Not Use
- When using this product
- Stop use and consult a doctor if
- If pregnant or breast feeding,
- Keep out of reach of children.
- Directions
-
Inactive ingredients
Water (Aqua), Coco-Caprylate/Caprate, Stearic Acid, PEG-8, Cetyl Alcohol, Stearyl Stearate, Glyceryl Stearate, PEG-100, Propylene Glycol, Kojic Acid, Daucus Carota Sative (Carrot) Seed Oil, Vaccinium Myrtillus Extract, Saccharum Officinarum (Sugar Cane) Extract, Acer Saccharum (Sugar Maple) Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Medica Limonum (Lemon) Fruit Extract, Pentetic Acid, Zea Mays (Corn) Oil, Xanthan Gum, Tocopheryl Acetate (Vitamin E), Retinyl Palmitate (Vitamin A), Beta-Carotene, Sodium Sulfite, Sodium Metabisulfite, Tetrasodium EDTA, Methylparaben, Propylparaben, DMDM Hydantoin, Fragrance (Parfum)
-
PRINCIPAL DISPLAY PANEL
Total White
Brightening Body Cream
Lightens Skin
Natural Bio-vegetal Activation
With Carrot Oil
8 OZ e 227 g Distributed by Heaven Scent, Miami FL
shimonb@yzyinc.com 877-999-3534
Tottenham, London N17 OQT, UK
MADE IN USA
Total White
Brightening Body Cream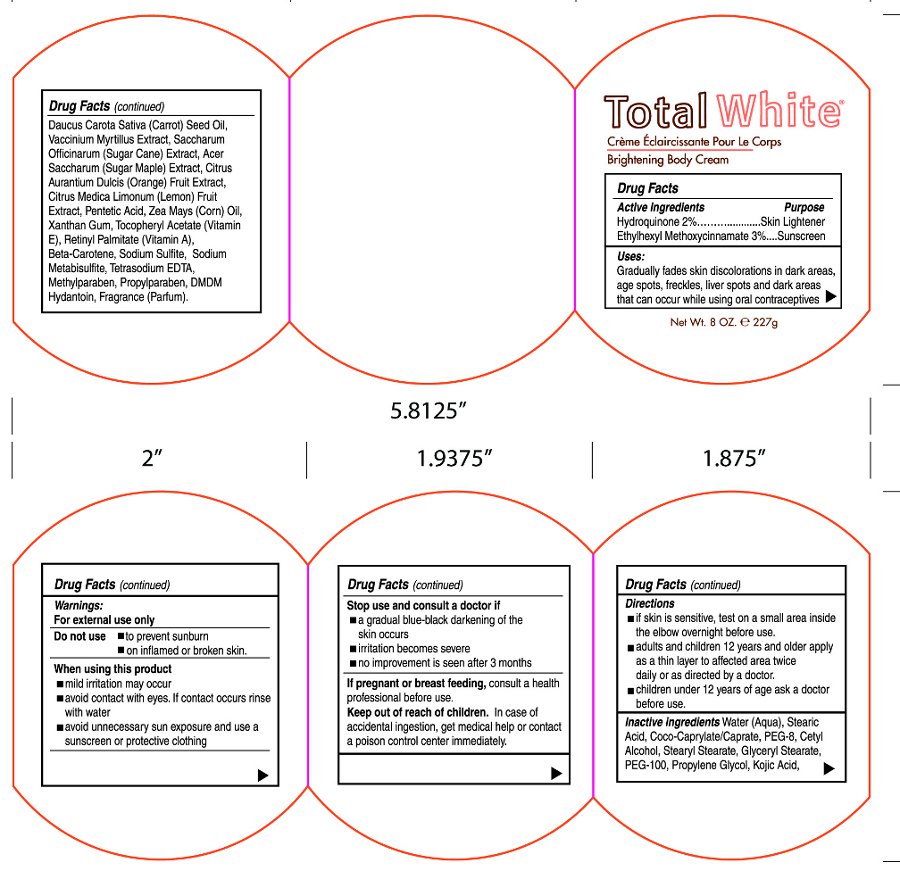
Total White Brightening Cream diminishes the appearance of dark spots and skin discolorations caused by acne, aging, sun damage and pregnancey. This rich cream is formulated with Carrot Oil and Vitamins A and E; it effectivly moisturizes and nourishes your skin, while it protects from future damage. It safely brightens and evens dark spots for younger, healthier, more radiant looking skin.

Total White Brightening Body Cream diminishes the appearance of dark spots and skin discolorations caused by acne, aging, sun damage, and pregnancy. This rich cream is formulated with Carrot Oil and Vitamins A and E, it effectively moisturizes and nourishes your skin, while it protects against future damage. It safely brightens and evens dark spots for younger, healthier more radiant looking skin.

Total White Brightening Body Cream With carrot oil Lightens SkinNatural Bio-Vegetal Activator

-
INGREDIENTS AND APPEARANCE
TOTAL WHITE BRIGHTENING
hydroquinone, ethylhexyl methoxycinnamate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53218-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 3 g in 100 g Hydroquinone (UNII: XV74C1N1AE) (Hydroquinone - UNII:XV74C1N1AE) Hydroquinone 2 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Coco-caprylate/caprate (UNII: 8D9H4QU99H) Stearic Acid (UNII: 4ELV7Z65AP) Polyethylene Glycol 400 (UNII: B697894SGQ) Cetyl Alcohol (UNII: 936JST6JCN) Stearyl Stearate (UNII: 5WX2EGD0DK) Glyceryl Monostearate (UNII: 230OU9XXE4) Propylene Glycol (UNII: 6DC9Q167V3) Kojic Acid (UNII: 6K23F1TT52) Carrot Seed Oil (UNII: 595AO13F11) Pentetic Acid (UNII: 7A314HQM0I) Corn Oil (UNII: 8470G57WFM) Xanthan Gum (UNII: TTV12P4NEE) .alpha.-tocopherol Acetate (UNII: 9E8X80D2L0) Vitamin A Palmitate (UNII: 1D1K0N0VVC) .beta.-carotene (UNII: 01YAE03M7J) Sodium Sulfite (UNII: VTK01UQK3G) Sodium Metabisulfite (UNII: 4VON5FNS3C) Edetate Sodium (UNII: MP1J8420LU) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Dmdm Hydantoin (UNII: BYR0546TOW) Polyethylene Glycol 4500 (UNII: TVH7653921) Sugarcane (UNII: 81H2R5AOH3) Orange (UNII: 5EVU04N5QU) Lemon (UNII: 24RS0A988O) Bilberry (UNII: 9P2U39H18W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53218-002-02 227 g in 1 JAR 2 NDC:53218-002-03 454 g in 1 JAR 3 NDC:53218-002-04 50 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/10/2012 Labeler - YZY Inc (174375659)