BICLORA - chlophedianol hydrochloride, chlorcyclizine hydrochloride liquid
Hawthorn Pharmaceuticals, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
biclora Liquid
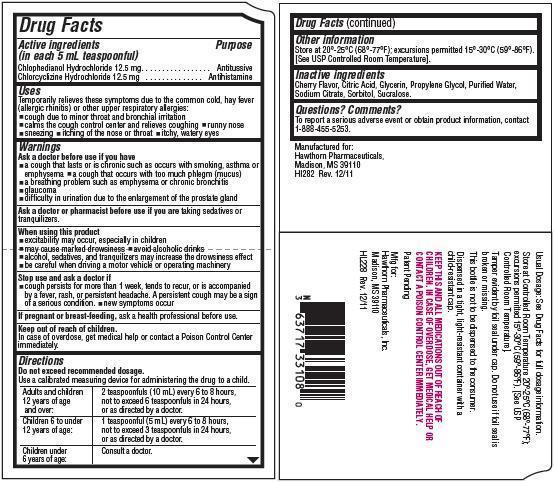
Drug Facts
Active ingredients
(in each 5 mL teaspoonful)
Chlophedianol Hydrochloride 12.5 mg
Chlorcyclizine Hydrochloride 12.5 mg
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- calms the cough control center and relieves coughing
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- be careful when driving a motor vehicle or operating machinery
Directions
Do not exceed recommended dosage.
Use a calibrated measuring device for administering the drug to a child.
| Adults and children 12 years of age and over: | 2 teaspoonfuls (10 mL) every 6 to 8 hours, not to exceed 6 teaspoonfuls in 24 hours, or as directed by a doctor. |
| Children 6 to under 12 years of age: | 1 teaspoonful (5 mL) every 6 to 8 hours, not to exceed 3 teaspoonfuls in 24 hours, or as directed by a doctor. |
| Children under 6 years of age: | Consult a doctor. |
Other information
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F).
[See USP Controlled Room Temperature].
Inactive ingredients
Cherry Flavor, Citric Acid, Glycerin, Propylene Glycol, Purified Water, Sodium Citrate, Sorbitol, Sucralsoe.
Questions? Comments?
To report a serious adverse event or obtain product information, contact
1-888-455-5253.
Manufactured for:
Hawthorn Pharmaceuticals,
Madison, MS 39110
HI282 Rev. 12/11
Product Packaging
The packaging below represents the labeling currently used.
Principal display panel for 8 fl oz. label:
NDC 63717-331-08
biclora LIQUID
Antitusive ∙ Antihistamine
Each teaspoonful (5 mL) contains:
Chlophedianol HCl.......................12.5 mg
Chlorcyclizine HCl.......................12.5 mg
For professional use.
The labeling for this product includes professional labeling
which is not intended for use by the general public.
Patent Pending
Alcohol Free ∙ Dye Free
Cherry Flavor
HAWTHORN
PHARMACEUTICALS, INC.
8 fl oz (240 mL)

| BICLORA
chlophedianol hydrochloride, chlorcyclizine hydrochloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Hawthorn Pharmaceuticals, Inc (118049704) |
| Registrant - Pernix Manufacturing, LLC (078641814) |