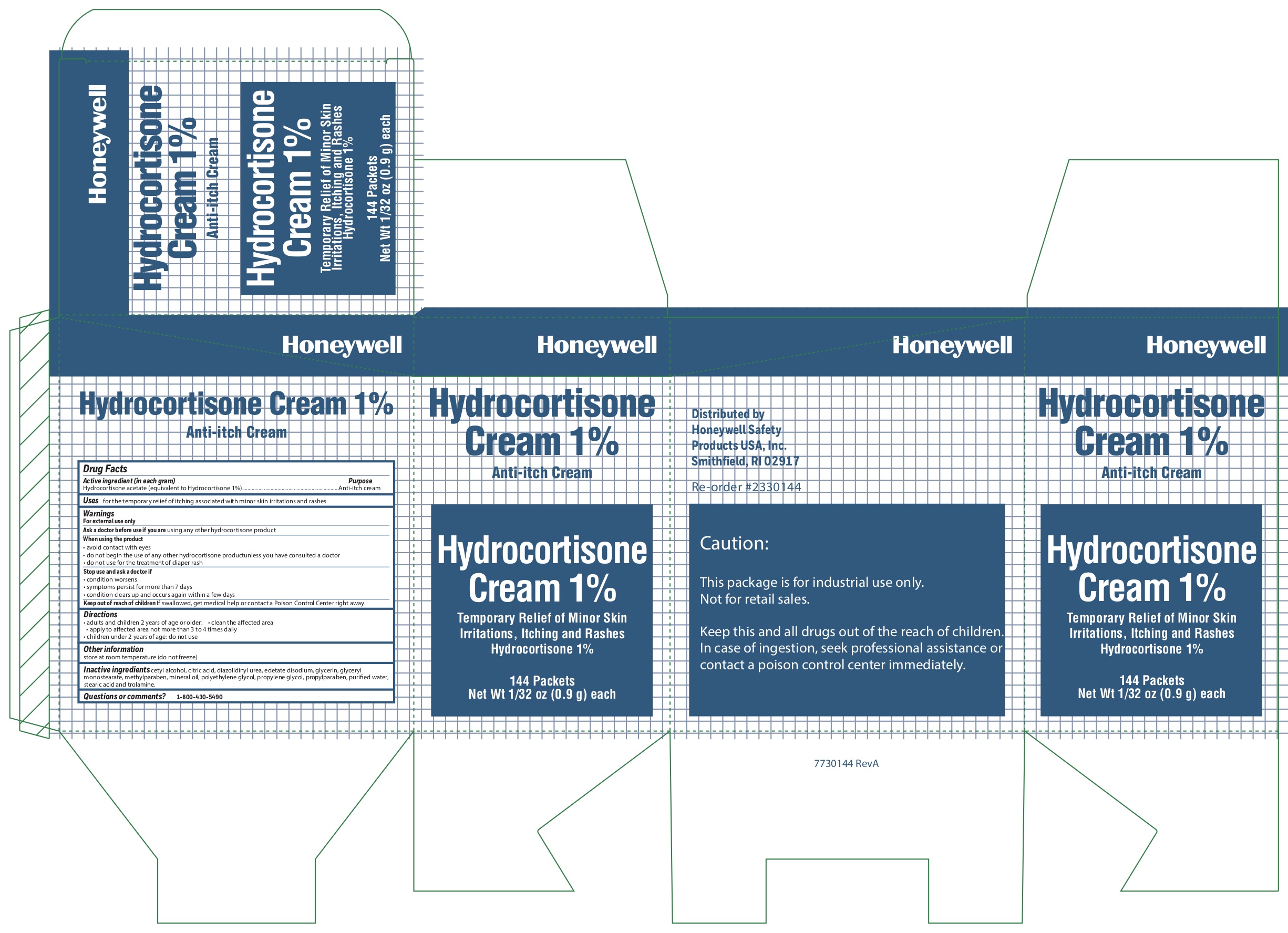
HYDROCORTISONE- anti-itch cream ointment
Honeywell Safety Products USA, inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-0800: 1% Hydrocortisone Cream
Warnings
For external use only
Ask a doctor before use if
Ask a doctor before use if you are using any other hydrocortisone product
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
Directions
- adults and children 2 years and older:
- clean the affected area
- apply to the area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
| HYDROCORTISONE
anti-itch cream ointment |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, inc (118768815) |
| Registrant - Honeywell Safety Products USA, Inc (118768815) |
Revised: 1/2024
Document Id: 0ec9b004-ecf8-99f2-e063-6394a90a6931
Set id: 51c4d717-c6c6-454d-af37-3145dffdba96
Version: 11
Effective Time: 20240111
Honeywell Safety Products USA, inc