Label: ZYVOX- linezolid tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-399-20 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0009-5135
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 22, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
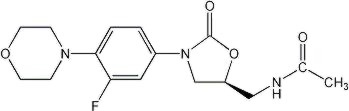
ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-acetamide.
The empirical formula is C16H20FN3O4. Its molecular weight is 337.35, and its chemical structure is represented below:
ZYVOX I.V. Injection is supplied as a ready-to-use sterile isotonic solution for intravenous infusion. Each mL contains 2 mg of linezolid. Inactive ingredients are sodium citrate, citric acid, and dextrose in an aqueous vehicle for intravenous administration. The sodium (Na+) content is 0.38 mg/mL (5 mEq per 300-mL bag; 3.3 mEq per 200-mL bag; and 1.7 mEq per 100-mL bag).
ZYVOX Tablets for oral administration contain 400 mg or 600 mg linezolid as film-coated compressed tablets. Inactive ingredients are corn starch, microcrystalline cellulose, hydroxypropylcellulose, sodium starch glycolate, magnesium stearate, hypromellose, polyethylene glycol, titanium dioxide, and carnauba wax. The sodium (Na+) content is 1.95 mg per 400-mg tablet and 2.92 mg per 600-mg tablet (0.1 mEq per tablet, regardless of strength).
ZYVOX for Oral Suspension is supplied as an orange-flavored granule/powder for constitution into a suspension for oral administration. Following constitution, each 5 mL contains 100 mg of linezolid. Inactive ingredients are sucrose, citric acid, sodium citrate, microcrystalline cellulose and carboxymethylcellulose sodium, aspartame, xanthan gum, mannitol, sodium benzoate, colloidal silicon dioxide, sodium chloride, and flavors (see PRECAUTIONS, Information for Patients). The sodium (Na+) content is 8.52 mg per 5 mL (0.4 mEq per 5 mL).
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
The mean pharmacokinetic parameters of linezolid in adults after single and multiple oral and intravenous (IV) doses are summarized in Table 1. Plasma concentrations of linezolid at steady-state after oral doses of 600 mg given every 12 hours (q12h) are shown in Figure 1.
Table 1. Mean (Standard Deviation) Pharmacokinetic Parameters of Linezolid in Adults Dose of Linezolid Cmax
µg/mLCmin
µg/mLTmax
hrsAUC *
µg ∙ h/mLt1/2
hrsCL
mL/minCmax = Maximum plasma concentration; Cmin = Minimum plasma concentration; Tmax = Time to Cmax; AUC = Area under concentration-time curve; t1/2 = Elimination half-life; CL = Systemic clearance 400 mg tablet
single dose †
every 12 hours
8.10
(1.83)
11.00
(4.37)
---
3.08
(2.25)
1.52
(1.01)
1.12
(0.47)
55.10
(25.00)
73.40
(33.50)
5.20
(1.50)
4.69
(1.70)
146
(67)
110
(49)600 mg tablet
single dose
every 12 hours
12.70
(3.96)
21.20
(5.78)
---
6.15
(2.94)
1.28
(0.66)
1.03
(0.62)
91.40
(39.30)
138.00
(42.10)
4.26
(1.65)
5.40
(2.06)
127
(48)
80
(29)600 mg IV injection‡
single dose
every 12 hours
12.90
(1.60)
15.10
(2.52)
---
3.68
(2.36)
0.50
(0.10)
0.51
(0.03)
80.20
(33.30)
89.70
(31.00)
4.40
(2.40)
4.80
(1.70)
138
(39)
123
(40)600 mg oral suspension
single dose
11.00
(2.76)
---
0.97
(0.88)
80.80
(35.10)
4.60
(1.71)
141
(45)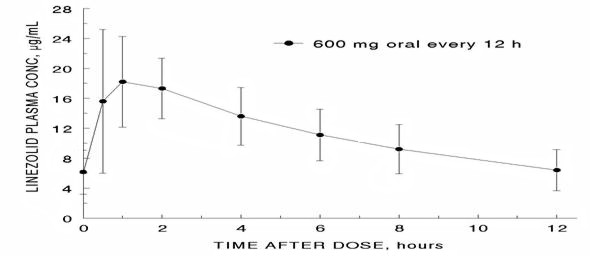
Figure 1. Plasma Concentrations of Linezolid in Adults at Steady-State Following Oral Dosing Every 12 Hours (Mean ± Standard Deviation, n=16)
Absorption
Linezolid is rapidly and extensively absorbed after oral dosing. Maximum plasma concentrations are reached approximately 1 to 2 hours after dosing, and the absolute bioavailability is approximately 100%. Therefore, linezolid may be given orally or intravenously without dose adjustment.
Linezolid may be administered without regard to the timing of meals. The time to reach the maximum concentration is delayed from 1.5 hours to 2.2 hours and Cmax is decreased by about 17% when high fat food is given with linezolid. However, the total exposure measured as AUC0-∞ values is similar under both conditions.
Distribution
Animal and human pharmacokinetic studies have demonstrated that linezolid readily distributes to well-perfused tissues. The plasma protein binding of linezolid is approximately 31% and is concentration-independent. The volume of distribution of linezolid at steady-state averaged 40 to 50 liters in healthy adult volunteers.
Linezolid concentrations have been determined in various fluids from a limited number of subjects in Phase 1 volunteer studies following multiple dosing of linezolid. The ratio of linezolid in saliva relative to plasma was 1.2 to 1 and for sweat relative to plasma was 0.55 to 1.
Metabolism
Linezolid is primarily metabolized by oxidation of the morpholine ring, which results in two inactive ring-opened carboxylic acid metabolites: the aminoethoxyacetic acid metabolite (A), and the hydroxyethyl glycine metabolite (B). Formation of metabolite A is presumed to be formed via an enzymatic pathway whereas metabolite B is mediated by a non-enzymatic chemical oxidation mechanism in vitro. In vitro studies have demonstrated that linezolid is minimally metabolized and may be mediated by human cytochrome P450. However, the metabolic pathway of linezolid is not fully understood.
Excretion
Nonrenal clearance accounts for approximately 65% of the total clearance of linezolid. Under steady-state conditions, approximately 30% of the dose appears in the urine as linezolid, 40% as metabolite B, and 10% as metabolite A. The renal clearance of linezolid is low (average 40 mL/min) and suggests net tubular reabsorption. Virtually no linezolid appears in the feces, while approximately 6% of the dose appears in the feces as metabolite B, and 3% as metabolite A.
A small degree of nonlinearity in clearance was observed with increasing doses of linezolid, which appears to be due to lower renal and nonrenal clearance of linezolid at higher concentrations. However, the difference in clearance was small and was not reflected in the apparent elimination half-life.
Special Populations
Geriatric
The pharmacokinetics of linezolid are not significantly altered in elderly patients (65 years or older). Therefore, dose adjustment for geriatric patients is not necessary.
Pediatric
The pharmacokinetics of linezolid following a single IV dose were investigated in pediatric patients ranging in age from birth through 17 years (including premature and full-term neonates), in healthy adolescent subjects ranging in age from 12 through 17 years, and in pediatric patients ranging in age from 1 week through 12 years. The pharmacokinetic parameters of linezolid are summarized in Table 2 for the pediatric populations studied and healthy adult subjects after administration of single IV doses.
The Cmax and the volume of distribution (Vss) of linezolid are similar regardless of age in pediatric patients. However, clearance of linezolid varies as a function of age. With the exclusion of pre-term neonates less than one week of age, clearance is most rapid in the youngest age groups ranging from >1 week old to 11 years, resulting in lower single-dose systemic exposure (AUC) and shorter half-life as compared with adults. As age of pediatric patients increases, the clearance of linezolid gradually decreases, and by adolescence mean clearance values approach those observed for the adult population. There is wider inter-subject variability in linezolid clearance and systemic drug exposure (AUC) across all pediatric age groups as compared with adults.
Similar mean daily AUC values were observed in pediatric patients from birth to 11 years of age dosed every 8 hours (q8h) relative to adolescents or adults dosed every 12 hours (q12h). Therefore, the dosage for pediatric patients up to 11 years of age should be 10 mg/kg q8h. Pediatric patients 12 years and older should receive 600 mg q12h (see DOSAGE AND ADMINISTRATION).
Table 2. Pharmacokinetic Parameters of Linezolid in Pediatrics and Adults Following a Single Intravenous Infusion of 10 mg/kg or 600 mg Linezolid (Mean: (%CV); [Min, Max Values])
Age GroupCmax
µg/mLVss
L/kgAUC *
µg • h/mLt 1/2
hrsCL
mL/min/kgCmax = Maximum plasma concentration; Vss= Volume of distribution; AUC = Area under concentration-time curve; t1/2 = Apparent elimination half-life; CL = Systemic clearance normalized for body weight - *
- AUC = Single dose AUC0–∞
- †
- In this data set, "pre-term" is defined as <34 weeks gestational age (Note: Only 1 patient enrolled was pre-term with a postnatal age between 1 week and 28 days)
- ‡
- Dose of 10 mg/kg
- §
- In this data set, "full-term" is defined as ≥34 weeks gestational age
- ¶
- Dose of 600 mg or 10 mg/kg up to a maximum of 600 mg
- #
- Dose normalized to 600 mg
Neonatal Patients
Pre-term†
< 1 week (N=9)‡
12.7 (30%)
[9.6, 22.2]
0.81 (24%)
[0.43, 1.05]
108 (47%)
[41, 191]
5.6 (46%)
[2.4, 9.8]
2.0 (52%)
[0.9, 4.0]Full-term§
< 1 week (N=10)‡
11.5 (24%)
[8.0, 18.3]
0.78 (20%)
[0.45, 0.96]
55 (47%)
[19, 103]
3.0 (55%)
[1.3, 6.1]
3.8 (55%)
[1.5, 8.8]Full-term§
≥ 1 week to ≤ 28 days (N=10)‡
12.9 (28%)
[7.7, 21.6]
0.66 (29%)
[0.35, 1.06]
34 (21%)
[23, 50]
1.5 (17%)
[1.2, 1.9]
5.1 (22%)
[3.3, 7.2]Infant Patients
> 28 days to < 3 Months (N=12 )‡
11.0 (27%)
[7.2, 18.0]
0.79 (26%)
[0.42, 1.08]
33 (26%)
[17, 48]
1.8 (28%)
[1.2, 2.8]
5.4 (32%)
[3.5, 9.9]Pediatric Patients
3 months through 11 years‡ (N=59)
15.1 (30%)
[6.8, 36.7]
0.69 (28%)
[0.31, 1.50]
58 (54%)
[19, 153]
2.9 (53%)
[0.9, 8.0]
3.8 (53%)
[1.0, 8.5]Adolescent Subjects and Patients
12 through 17 years¶ (N=36)
16.7 (24%)
[9.9, 28.9]
0.61 (15%)
[0.44, 0.79]
95 (44%)
[32, 178]
4.1 (46%)
[1.3, 8.1]
2.1 (53%)
[0.9, 5.2]Adult Subjects# (N= 29) 12.5 (21%)
[8.2, 19.3]0.65 (16%)
[0.45, 0.84]91 (33%)
[53, 155]4.9 (35%)
[1.8, 8.3]1.7 (34%)
[0.9, 3.3]Gender
Females have a slightly lower volume of distribution of linezolid than males. Plasma concentrations are higher in females than in males, which is partly due to body weight differences. After a 600-mg dose, mean oral clearance is approximately 38% lower in females than in males. However, there are no significant gender differences in mean apparent elimination-rate constant or half-life. Thus, drug exposure in females is not expected to substantially increase beyond levels known to be well tolerated. Therefore, dose adjustment by gender does not appear to be necessary.
Renal Insufficiency
The pharmacokinetics of the parent drug, linezolid, are not altered in patients with any degree of renal insufficiency; however, the two primary metabolites of linezolid may accumulate in patients with renal insufficiency, with the amount of accumulation increasing with the severity of renal dysfunction (see Table 3). The clinical significance of accumulation of these two metabolites has not been determined in patients with severe renal insufficiency. Because similar plasma concentrations of linezolid are achieved regardless of renal function, no dose adjustment is recommended for patients with renal insufficiency. However, given the absence of information on the clinical significance of accumulation of the primary metabolites, use of linezolid in patients with renal insufficiency should be weighed against the potential risks of accumulation of these metabolites. Both linezolid and the two metabolites are eliminated by dialysis. No information is available on the effect of peritoneal dialysis on the pharmacokinetics of linezolid. Approximately 30% of a dose was eliminated in a 3-hour dialysis session beginning 3 hours after the dose of linezolid was administered; therefore, linezolid should be given after hemodialysis.
Table 3. Mean (Standard Deviation) AUCs and Elimination Half-lives of Linezolid and Metabolites A and B in Patients with Varying Degrees of Renal Insufficiency After a Single 600-mg Oral Dose of Linezolid Parameter Healthy Subjects CLCR > 80 mL/min Moderate Renal Impairment 30 < CLCR < 80 mL/min Severe Renal Impairment 10 < CLCR < 30 mL/min Hemodialysis-Dependent Off Dialysis* On Dialysis NA = Not applicable - *
- between hemodialysis sessions
Linezolid AUC0–∞, µg h/mL 110 (22) 128 (53) 127 (66) 141 (45) 83 (23) t1/2, hours 6.4 (2.2) 6.1 (1.7) 7.1 (3.7) 8.4 (2.7) 7.0 (1.8) Metabolite A AUC0–48, µg h/mL 7.6 (1.9) 11.7 (4.3) 56.5 (30.6) 185 (124) 68.8 (23.9) t1/2, hours 6.3 (2.1) 6.6 (2.3) 9.0 (4.6) NA NA Metabolite B AUC0–48, µg h/mL 30.5 (6.2) 51.1 (38.5) 203 (92) 467 (102) 239 (44) t1/2, hours 6.6 (2.7) 9.9 (7.4) 11.0 (3.9) NA NA Hepatic Insufficiency
The pharmacokinetics of linezolid are not altered in patients (n=7) with mild-to-moderate hepatic insufficiency (Child-Pugh class A or B). On the basis of the available information, no dose adjustment is recommended for patients with mild-to-moderate hepatic insufficiency. The pharmacokinetics of linezolid in patients with severe hepatic insufficiency have not been evaluated.
Drug-Drug Interactions
Drugs Metabolized by Cytochrome P450
Linezolid is not an inducer of cytochrome P450 (CYP450) in rats. In addition, linezolid does not inhibit the activities of clinically significant human CYP isoforms (e.g., 1A2, 2C9, 2C19, 2D6, 2E1, 3A4). Therefore, linezolid is not expected to affect the pharmacokinetics of other drugs metabolized by these major enzymes. Concurrent administration of linezolid does not substantially alter the pharmacokinetic characteristics of (S)-warfarin, which is extensively metabolized by CYP2C9. Drugs such as warfarin and phenytoin, which are CYP2C9 substrates, may be given with linezolid without changes in dosage regimen.
Antibiotics
Aztreonam: The pharmacokinetics of linezolid or aztreonam are not altered when administered together.
Gentamicin: The pharmacokinetics of linezolid or gentamicin are not altered when administered together.
Rifampin: The effect of rifampin on the pharmacokinetics of linezolid was evaluated in a study of 16 healthy adult males. Volunteers were administered oral linezolid 600 mg twice daily for 5 doses with and without rifampin 600 mg once daily for 8 days. Co-administration of rifampin with linezolid resulted in a 21% decrease in linezolid Cmax [90% CI, 15% – 27%] and a 32% decrease in linezolid AUC0–12 [90% CI, 27% – 37%]. The mechanism of this interaction is not fully understood and may be related to the induction of hepatic enzymes (see PRECAUTIONS, Drug Interactions).
Monoamine Oxidase Inhibition
Linezolid is a reversible, nonselective inhibitor of monoamine oxidase. Therefore, linezolid has the potential for interaction with adrenergic and serotonergic agents.
Adrenergic Agents: A significant pressor response has been observed in normal adult subjects receiving linezolid and tyramine doses of more than 100 mg. Therefore, patients receiving linezolid need to avoid consuming large amounts of foods or beverages with high tyramine content (see PRECAUTIONS, Information for Patients).
A reversible enhancement of the pressor response of either pseudoephedrine HCl (PSE) or phenylpropanolamine HCl (PPA) is observed when linezolid is administered to healthy normotensive subjects (see PRECAUTIONS, Drug Interactions). A similar study has not been conducted in hypertensive patients. The interaction studies conducted in normotensive subjects evaluated the blood pressure and heart rate effects of placebo, PPA or PSE alone, linezolid alone, and the combination of steady-state linezolid (600 mg q12h for 3 days) with two doses of PPA (25 mg) or PSE (60 mg) given 4 hours apart. Heart rate was not affected by any of the treatments. Blood pressure was increased with both combination treatments. Maximum blood pressure levels were seen 2 to 3 hours after the second dose of PPA or PSE, and returned to baseline 2 to 3 hours after peak. The results of the PPA study follow, showing the mean (and range) maximum systolic blood pressure in mm Hg: placebo = 121 (103 to 158); linezolid alone = 120 (107 to 135); PPA alone = 125 (106 to 139); PPA with linezolid = 147 (129 to 176). The results from the PSE study were similar to those in the PPA study. The mean maximum increase in systolic blood pressure over baseline was 32 mm Hg (range: 20–52 mm Hg) and 38 mm Hg (range: 18–79 mm Hg) during co-administration of linezolid with pseudoephedrine or phenylpropanolamine, respectively.
Serotonergic Agents: The potential drug-drug interaction with dextromethorphan was studied in healthy volunteers. Subjects were administered dextromethorphan (two 20-mg doses given 4 hours apart) with or without linezolid. No serotonin syndrome effects (confusion, delirium, restlessness, tremors, blushing, diaphoresis, hyperpyrexia) have been observed in normal subjects receiving linezolid and dextromethorphan.
MICROBIOLOGY
Linezolid is a synthetic antibacterial agent of a new class of antibiotics, the oxazolidinones, which has clinical utility in the treatment of infections caused by aerobic Gram-positive bacteria. The in vitro spectrum of activity of linezolid also includes certain Gram-negative bacteria and anaerobic bacteria. Linezolid inhibits bacterial protein synthesis through a mechanism of action different from that of other antibacterial agents; therefore, cross-resistance between linezolid and other classes of antibiotics is unlikely. Linezolid binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, which is an essential component of the bacterial translation process. The results of time-kill studies have shown linezolid to be bacteriostatic against enterococci and staphylococci. For streptococci, linezolid was found to be bactericidal for the majority of strains.
In clinical trials, resistance to linezolid developed in 6 patients infected with Enterococcus faecium (4 patients received 200 mg q12h, lower than the recommended dose, and 2 patients received 600 mg q12h). In a compassionate use program, resistance to linezolid developed in 8 patients with E. faecium and in 1 patient with Enterococcus faecalis. All patients had either unremoved prosthetic devices or undrained abscesses. Resistance to linezolid occurs in vitro at a frequency of 1 x 10 -9 to 1 x 10 -11. In vitro studies have shown that point mutations in the 23S rRNA are associated with linezolid resistance. Reports of vancomycin-resistant E. faecium becoming resistant to linezolid during its clinical use have been published.1 In one report nosocomial spread of vancomycin- and linezolid-resistant E. faecium occurred 2. There has been a report of Staphylococcus aureus (methicillin-resistant) developing resistance to linezolid during its clinical use.3 The linezolid resistance in these organisms was associated with a point mutation in the 23S rRNA (substitution of thymine for guanine at position 2576) of the organism. When antibiotic-resistant organisms are encountered in the hospital, it is important to emphasize infection control policies.4, 5 Resistance to linezolid has not been reported in Streptococcus spp., including Streptococcus pneumoniae.
In vitro studies have demonstrated additivity or indifference between linezolid and vancomycin, gentamicin, rifampin, imipenem-cilastatin, aztreonam, ampicillin, or streptomycin.
Linezolid has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections, as described in the INDICATIONS AND USAGE section.
Aerobic and facultative Gram-positive microorganisms
Enterococcus faecium (vancomycin-resistant strains only)
Staphylococcus aureus (including methicillin-resistant strains)
Streptococcus agalactiae
Streptococcus pneumoniae (including multi-drug resistant isolates [MDRSP]1)
Streptococcus pyogenesThe following in vitro data are available, but their clinical significance is unknown. At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for linezolid. However, the safety and effectiveness of linezolid in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
- 1
-
MDRSP refers to isolates resistant to two or more of the following antibiotics: penicillin, second-generation cephalosporins, macrolides, tetracycline, and trimethoprim/sulfamethoxazole.
Aerobic and facultative Gram-positive microorganisms
Enterococcus faecalis (including vancomycin-resistant strains)
Enterococcus faecium (vancomycin-susceptible strains)
Staphylococcus epidermidis (including methicillin-resistant strains)
Staphylococcus haemolyticus
Viridans group streptococciSusceptibility Testing Methods
NOTE: Susceptibility testing by dilution methods requires the use of linezolid susceptibility powder.
When available, the results of in vitro susceptibility tests should be provided to the physician as periodic reports which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method 6,7 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of linezolid powder. The MIC values should be interpreted according to criteria provided in Table 4.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure 7,8 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 µg of linezolid to test the susceptibility of microorganisms to linezolid. The disk diffusion interpretive criteria are provided in Table 4.
Table 4. Susceptibility Interpretive Criteria for Linezolid Pathogen Susceptibility Interpretive Criteria Minimal Inhibitory Concentrations
(MIC in µg/mL)Disk Diffusion
(Zone Diameters in mm)S I R S I R - *
- The current absence of data on resistant strains precludes defining any categories other than "Susceptible." Strains yielding test results suggestive of a "nonsusceptible" category should be retested, and if the result is confirmed, the isolate should be submitted to a reference laboratory for further testing.
- †
- These interpretive standards for S. pneumoniae and Streptococcus spp. other than S. pneumoniae are applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35°C for 20 to 24 hours.
- ‡
- These zone diameter interpretive standards are applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35°C for 20 to 24 hours.
Enterococcus spp ≤ 2 4 ≥8 ≥ 23 21–22 ≤20 Staphylococcus spp * ≤4 --- --- ≥ 21 --- --- Streptococcus pneumoniae* ≤2† --- --- ≥ 21‡ --- --- Streptococcus spp other than S pneumoniae* ≤2† --- --- ≥ 21‡ --- --- A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of quality control microorganisms to control the technical aspects of the test procedures. Standard linezolid powder should provide the following range of values noted in Table 5. NOTE: Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within bacteria; the specific strains used for microbiological quality control are not clinically significant.
Table 5. Acceptable Quality Control Ranges for Linezolid to be Used in Validation of Susceptibility Test Results QC Strain Acceptable Quality Control Ranges Minimum Inhibitory Concentration
(MIC in µg/mL)Disk Diffusion
(Zone Diameters in mm)- *
- This organism may be used for validation of susceptibility test results when testing Streptococcus spp. other than S. pneumoniae.
- †
- This quality control range for S. pneumoniae is applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35°C for 20 to 24 hours.
- ‡
- This quality control zone diameter range is applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35°C for 20 to 24 hours.
Enterococcus faecalis
ATCC 292121 – 4 Not applicable Staphylococcus aureus
ATCC 292131 – 4 Not applicable Staphylococcus aureus
ATCC 25923Not applicable 25 – 32 Streptococcus pneumoniae
ATCC 49619*0.50 – 2† 25 – 34‡ -
INDICATIONS AND USAGE
ZYVOX formulations are indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms (see PRECAUTIONS, Pediatric Use and DOSAGE AND ADMINISTRATION and CLINICAL STUDIES). Linezolid is not indicated for the treatment of Gram-negative infections. It is critical that specific Gram-negative therapy be initiated immediately if a concomitant Gram-negative pathogen is documented or suspected (see WARNINGS).
Vancomycin-Resistant Enterococcus faecium infections, including cases with concurrent bacteremia. (see CLINICAL STUDIES)
Nosocomial pneumonia caused by Staphylococcus aureus (methicillin-susceptible and -resistant strains), or Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP]).
Complicated skin and skin structure infections, including diabetic foot infections, without concomitant osteomyelitis, caused by Staphylococcus aureus (methicillin-susceptible and -resistant strains), Streptococcus pyogenes, or Streptococcus agalactiae. ZYVOX has not been studied in the treatment of decubitus ulcers.
Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible only) or Streptococcus pyogenes.
Community-acquired pneumonia caused by Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP]2), including cases with concurrent bacteremia, or Staphylococcus aureus (methicillin-susceptible strains only).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZYVOX and other antibacterial drugs, ZYVOX should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- 2
-
MDRSP refers to isolates resistant to two or more of the following antibiotics: penicillin, second-generation cephalosporins, macrolides, tetracycline, and trimethoprim/sulfamethoxazole.
-
CONTRAINDICATIONS
ZYVOX formulations are contraindicated for use in patients who have known hypersensitivity to linezolid or any of the other product components.
Monoamine Oxidase Inhibitors
Linezolid should not be used in patients taking any medicinal product which inhibits monoamine oxidases A or B (e.g., phenelzine, isocarboxazid) or within two weeks of taking any such medicinal product.
Potential Interactions Producing Elevation of Blood Pressure
Unless patients are monitored for potential increases in blood pressure, linezolid should not be administered to patients with uncontrolled hypertension, pheochromocytoma, thyrotoxicosis and/or patients taking any of the following types of medications: directly and indirectly acting sympathomimetic agents (e.g., pseudoephedrine), vasopressive agents (e.g., epinephrine, norepinephrine), dopaminergic agents (e.g., dopamine, dobutamine) (see PRECAUTIONS, Drug Interactions).
Potential Serotonergic Interactions
Unless patients are carefully observed for signs and/or symptoms of serotonin syndrome, linezolid should not be administered to patients with carcinoid syndrome and/or patients taking any of the following medications: serotonin re-uptake inhibitors, tricyclic antidepressants, serotonin 5-HT1 receptor agonists (triptans), meperidine or buspirone (see PRECAUTIONS, General and Drug Interactions).
-
WARNINGS
Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia) has been reported in patients receiving linezolid. In cases where the outcome is known, when linezolid was discontinued, the affected hematologic parameters have risen toward pretreatment levels. Complete blood counts should be monitored weekly in patients who receive linezolid, particularly in those who receive linezolid for longer than two weeks, those with pre-existing myelosuppression, those receiving concomitant drugs that produce bone marrow suppression, or those with a chronic infection who have received previous or concomitant antibiotic therapy. Discontinuation of therapy with linezolid should be considered in patients who develop or have worsening myelosuppression.
In adult and juvenile dogs and rats, myelosuppression, reduced extramedullary hematopoiesis in spleen and liver, and lymphoid depletion of thymus, lymph nodes, and spleen were observed (see ANIMAL PHARMACOLOGY).
Mortality Imbalance in an Investigational Study in Patients with Catheter-Related Bloodstream Infections, including those with catheter-site infections
An imbalance in mortality was seen in patients treated with linezolid relative to vancomycin/dicloxacillin/oxacillin in an open-label study in seriously ill patients with intravascular catheter-related infections [78/363 (21.5%) vs. 58/363 (16.0%); odds ratio 1.426, 95% CI 0.970, 2.098]. While causality has not been established, this observed imbalance occurred primarily in linezolid-treated patients in whom either Gram-negative pathogens, mixed Gram-negative and Gram-positive pathogens, or no pathogen were identified at baseline, but was not seen in patients with Gram-positive infections only.
Linezolid is not approved and should not be used for the treatment of patients with catheter-related bloodstream infections or catheter-site infections.
Linezolid has no clinical activity against Gram-negative pathogens and is not indicated for the treatment of Gram-negative infections. It is critical that specific Gram-negative therapy be initiated immediately if a concomitant Gram-negative pathogen is documented or suspected (see INDICATIONS AND USAGE).
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ZYVOX, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use.
Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Lactic Acidosis
Lactic acidosis has been reported with the use of ZYVOX. In reported cases, patients experienced repeated episodes of nausea and vomiting. Patients who develop recurrent nausea or vomiting, unexplained acidosis, or a low bicarbonate level while receiving ZYVOX should receive immediate medical evaluation.
Serotonin Syndrome
Spontaneous reports of serotonin syndrome associated with the co-administration of ZYVOX and serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs), have been reported (see PRECAUTIONS, Drug Interactions).
Where administration of ZYVOX and concomitant serotonergic agents is clinically appropriate, patients should be closely observed for signs and symptoms of serotonin syndrome such as cognitive dysfunction, hyperpyrexia, hyperreflexia and incoordination. If signs or symptoms occur physicians should consider discontinuation of either one or both agents. If the concomitant serotonergic agent is withdrawn, discontinuation symptoms can be observed (see package insert of the specified agent(s) for a description of the associated discontinuation symptoms).
Peripheral and Optic Neuropathy
Peripheral and optic neuropathy have been reported in patients treated with ZYVOX, primarily those patients treated for longer than the maximum recommended duration of 28 days. In cases of optic neuropathy that progressed to loss of vision, patients were treated for extended periods beyond the maximum recommended duration. Visual blurring has been reported in some patients treated with ZYVOX for less than 28 days.
If patients experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, blurred vision, or visual field defect, prompt ophthalmic evaluation is recommended. Visual function should be monitored in all patients taking ZYVOX for extended periods (≥ 3 months) and in all patients reporting new visual symptoms regardless of length of therapy with ZYVOX. If peripheral or optic neuropathy occurs, the continued use of ZYVOX in these patients should be weighed against the potential risks.
Convulsions
Convulsions have been reported in patients when treated with linezolid. In some of these cases, a history of seizures or risk factors for seizures was reported.
The use of antibiotics may promote the overgrowth of nonsusceptible organisms. Should superinfection occur during therapy, appropriate measures should be taken.
ZYVOX has not been studied in patients with uncontrolled hypertension, pheochromocytoma, carcinoid syndrome, or untreated hyperthyroidism.
The safety and efficacy of ZYVOX formulations given for longer than 28 days have not been evaluated in controlled clinical trials.
Prescribing ZYVOX in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be advised that:
- ZYVOX may be taken with or without food.
- They should inform their physician if they have a history of hypertension.
- Large quantities of foods or beverages with high tyramine content should be avoided while taking ZYVOX. Quantities of tyramine consumed should be less than 100 mg per meal. Foods high in tyramine content include those that may have undergone protein changes by aging, fermentation, pickling, or smoking to improve flavor, such as aged cheeses (0 to 15 mg tyramine per ounce); fermented or air-dried meats (0.1 to 8 mg tyramine per ounce); sauerkraut (8 mg tyramine per 8 ounces); soy sauce (5 mg tyramine per 1 teaspoon); tap beers (4 mg tyramine per 12 ounces); red wines (0 to 6 mg tyramine per 8 ounces). The tyramine content of any protein-rich food may be increased if stored for long periods or improperly refrigerated.9,10
- They should inform their physician if taking medications containing pseudoephedrine HCl or phenylpropanolamine HCl, such as cold remedies and decongestants.
- They should inform their physician if taking serotonin re-uptake inhibitors or other antidepressants.
- Phenylketonurics: Each 5 mL of the 100 mg/5 mL ZYVOX for Oral Suspension contains 20 mg phenylalanine. The other ZYVOX formulations do not contain phenylalanine. Contact your physician or pharmacist.
- They should inform their physician if they experience changes in vision.
- They should inform their physician if they have a history of seizures.
- Diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Patients should be counseled that antibacterial drugs including ZYVOX should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ZYVOX is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ZYVOX or other antibacterial drugs in the future.
Drug Interactions
(see also CLINICAL PHARMACOLOGY, Drug-Drug Interactions)
Monoamine Oxidase Inhibition
Linezolid is a reversible, nonselective inhibitor of monoamine oxidase. Therefore, linezolid has the potential for interaction with adrenergic and serotonergic agents.
Adrenergic Agents: Some individuals receiving ZYVOX may experience a reversible enhancement of the pressor response to indirect-acting sympathomimetic agents, vasopressor or dopaminergic agents. Commonly used drugs such as phenylpropanolamine and pseudoephedrine have been specifically studied. Initial doses of adrenergic agents, such as dopamine or epinephrine, should be reduced and titrated to achieve the desired response.
Serotonergic Agents: Co-administration of linezolid and serotonergic agents was not associated with serotonin syndrome in Phase 1, 2 or 3 studies. Spontaneous reports of serotonin syndrome associated with co-administration of ZYVOX and serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs), have been reported. Patients who are treated with ZYVOX and concomitant serotonergic agents should be closely observed as described in the PRECAUTIONS, General Section.
Strong CYP450 Inducers: In a study in healthy volunteers, co-administration of rifampin with oral linezolid resulted in a 21% decrease in linezolid Cmax and a 32% decrease in linezolid AUC0–12. The clinical significance of this interaction is unknown. Other strong inducers of hepatic enzymes (e.g. carbamazepine, phenytoin, phenobarbital) could cause a similar or smaller decrease in linezolid exposure (see CLINICAL PHARMACOLOGY, Drug-Drug Interactions)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime studies in animals have not been conducted to evaluate the carcinogenic potential of linezolid. Neither mutagenic nor clastogenic potential was found in a battery of tests including: assays for mutagenicity (Ames bacterial reversion and CHO cell mutation), an in vitro unscheduled DNA synthesis (UDS) assay, an in vitro chromosome aberration assay in human lymphocytes, and an in vivo mouse micronucleus assay.
Linezolid did not affect the fertility or reproductive performance of adult female rats. It reversibly decreased fertility and reproductive performance in adult male rats when given at doses ≥ 50 mg/kg/day, with exposures approximately equal to or greater than the expected human exposure level (exposure comparisons are based on AUCs). The reversible fertility effects were mediated through altered spermatogenesis. Affected spermatids contained abnormally formed and oriented mitochondria and were non-viable. Epithelial cell hypertrophy and hyperplasia in the epididymis was observed in conjunction with decreased fertility. Similar epididymal changes were not seen in dogs.
In sexually mature male rats exposed to drug as juveniles, mildly decreased fertility was observed following treatment with linezolid through most of their period of sexual development (50 mg/kg/day from days 7 to 36 of age, and 100 mg/kg/day from days 37 to 55 of age), with exposures up to 1.7-fold greater than mean AUCs observed in pediatric patients aged 3 months to 11 years. Decreased fertility was not observed with shorter treatment periods, corresponding to exposure in utero through the early neonatal period (gestation day 6 through postnatal day 5), neonatal exposure (postnatal days 5 to 21), or to juvenile exposure (postnatal days 22 to 35). Reversible reductions in sperm motility and altered sperm morphology were observed in rats treated from postnatal day 22 to 35.
Pregnancy
Teratogenic Effects.
Pregnancy Category C
Linezolid was not teratogenic in mice, rats, or rabbits at exposure levels 6.5-fold (in mice), equivalent to (in rats), or 0.06-fold (in rabbits) the expected human exposure level, based on AUCs. However, embryo and fetal toxicities were seen (see Non-teratogenic Effects). There are no adequate and well-controlled studies in pregnant women. ZYVOX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-teratogenic Effects
In mice, embryo and fetal toxicities were seen only at doses that caused maternal toxicity (clinical signs and reduced body weight gain). A dose of 450 mg/kg/day (6.5-fold the estimated human exposure level based on AUCs) correlated with increased postimplantational embryo death, including total litter loss, decreased fetal body weights, and an increased incidence of costal cartilage fusion.
In rats, mild fetal toxicity was observed at 15 and 50 mg/kg/day (exposure levels 0.22-fold to approximately equivalent to the estimated human exposure, respectively based on AUCs). The effects consisted of decreased fetal body weights and reduced ossification of sternebrae, a finding often seen in association with decreased fetal body weights. Slight maternal toxicity, in the form of reduced body weight gain, was seen at 50 mg/kg/day.
In rabbits, reduced fetal body weight occurred only in the presence of maternal toxicity (clinical signs, reduced body weight gain and food consumption) when administered at a dose of 15 mg/kg/day (0.06-fold the estimated human exposure based on AUCs).
When female rats were treated with 50 mg/kg/day (approximately equivalent to the estimated human exposure based on AUCs) of linezolid during pregnancy and lactation, survival of pups was decreased on postnatal days 1 to 4. Male and female pups permitted to mature to reproductive age, when mated, showed an increase in preimplantation loss.
Nursing Mothers
Linezolid and its metabolites are excreted in the milk of lactating rats. Concentrations in milk were similar to those in maternal plasma. It is not known whether linezolid is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ZYVOX is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of ZYVOX for the treatment of pediatric patients with the following infections are supported by evidence from adequate and well-controlled studies in adults, pharmacokinetic data in pediatric patients, and additional data from a comparator-controlled study of Gram-positive infections in pediatric patients ranging in age from birth through 11 years (see INDICATIONS AND USAGE and CLINICAL STUDIES):
- nosocomial pneumonia
- complicated skin and skin structure infections
- community-acquired pneumonia (also supported by evidence from an uncontrolled study in patients ranging in age from 8 months through 12 years)
- vancomycin-resistant Enterococcus faecium infections
The safety and effectiveness of ZYVOX for the treatment of pediatric patients with the following infection have been established in a comparator-controlled study in pediatric patients ranging in age from 5 through 17 years (see CLINICAL STUDIES):
- uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes
Pharmacokinetic information generated in pediatric patients with ventriculoperitoneal shunts showed variable cerebrospinal fluid (CSF) linezolid concentrations following single and multiple dosing of linezolid; therapeutic concentrations were not consistently achieved or maintained in the CSF. Therefore, the use of linezolid for the empiric treatment of pediatric patients with central nervous system infections is not recommended.
The Cmax and the volume of distribution (Vss) of linezolid are similar regardless of age in pediatric patients. However, linezolid clearance is a function of age. Excluding neonates less than a week of age, clearance is most rapid in the youngest age groups ranging from >1 week old to 11 years, resulting in lower single-dose systemic exposure (AUC) and shorter half-life as compared with adults. As age of pediatric patients increases, the clearance of linezolid gradually decreases, and by adolescence, mean clearance values approach those observed for the adult population. There is wider inter-subject variability in linezolid clearance and in systemic drug exposure (AUC) across all pediatric age groups as compared with adults.
Similar mean daily AUC values were observed in pediatric patients from birth to 11 years of age dosed q8h relative to adolescents or adults dosed q12h. Therefore, the dosage for pediatric patients up to 11 years of age should be 10 mg/kg q8h. Pediatric patients 12 years and older should receive 600 mg q12h.
Recommendations for the dosage regimen for pre-term neonates less than 7 days of age (gestational age less than 34 weeks) are based on pharmacokinetic data from 9 pre-term neonates. Most of these pre-term neonates have lower systemic linezolid clearance values and larger AUC values than many full-term neonates and older infants. Therefore, these pre-term neonates should be initiated with a dosing regimen of 10 mg/kg q12h. Consideration may be given to the use of a 10 mg/kg q8h regimen in neonates with a sub-optimal clinical response. All neonatal patients should receive 10 mg/kg q8h by 7 days of life (see CLINICAL PHARMACOLOGY, Special Populations, Pediatric and DOSAGE AND ADMINISTRATION ).
In limited clinical experience, 5 out of 6 (83%) pediatric patients with infections due to Gram-positive pathogens with MICs of 4 µg/mL treated with ZYVOX had clinical cures. However, pediatric patients exhibit wider variability in linezolid clearance and systemic exposure (AUC) compared with adults. In pediatric patients with a sub-optimal clinical response, particularly those with pathogens with MIC of 4 µg/mL, lower systemic exposure, site and severity of infection, and the underlying medical condition should be considered when assessing clinical response (see CLINICAL PHARMACOLOGY, Special Populations, Pediatric and DOSAGE AND ADMINISTRATION).
-
ANIMAL PHARMACOLOGY
Target organs of linezolid toxicity were similar in juvenile and adult rats and dogs. Dose- and time-dependent myelosuppression, as evidenced by bone marrow hypocellularity/decreased hematopoiesis, decreased extramedullary hematopoiesis in spleen and liver, and decreased levels of circulating erythrocytes, leukocytes, and platelets have been seen in animal studies. Lymphoid depletion occurred in thymus, lymph nodes, and spleen. Generally, the lymphoid findings were associated with anorexia, weight loss, and suppression of body weight gain, which may have contributed to the observed effects.
In rats administered linezolid orally for 6 months, non-reversible, minimal to mild axonal degeneration of sciatic nerves was observed at 80 mg/kg/day; minimal degeneration of the sciatic nerve was also observed in 1 male at this dose level at a 3-month interim necropsy. Sensitive morphologic evaluation of perfusion-fixed tissues was conducted to investigate evidence of optic nerve degeneration. Minimal to moderate optic nerve degeneration was evident in 2 male rats after 6 months of dosing, but the direct relationship to drug was equivocal because of the acute nature of the finding and its asymmetrical distribution. The nerve degeneration observed was microscopically comparable to spontaneous unilateral optic nerve degeneration reported in aging rats and may be an exacerbation of common background change.
These effects were observed at exposure levels that are comparable to those observed in some human subjects. The hematopoietic and lymphoid effects were reversible, although in some studies, reversal was incomplete within the duration of the recovery period.
-
ADVERSE REACTIONS
Adult Patients
The safety of ZYVOX formulations was evaluated in 2046 adult patients enrolled in seven Phase 3 comparator-controlled clinical trials, who were treated for up to 28 days. In these studies, 85% of the adverse events reported with ZYVOX were described as mild to moderate in intensity. Table 6 shows the incidence of adverse events reported in at least 2% of patients in these trials. The most common adverse events in patients treated with ZYVOX were diarrhea (incidence across studies: 2.8% to 11.0%), headache (incidence across studies: 0.5% to 11.3%), and nausea (incidence across studies: 3.4% to 9.6%).
Table 6. Incidence (%) of Adverse Events Reported in ≥2% of Adult Patients in Comparator-Controlled Clinical Trials with ZYVOX Event ZYVOX
(n=2046)All Comparators *
(n=2001)- *
- Comparators included cefpodoxime proxetil 200 mg PO q12h; ceftriaxone 1 g IV q12h; clarithromycin 250 mg PO q12h; dicloxacillin 500 mg PO q6h; oxacillin 2 g IV q6h; vancomycin 1 g IV q12h.
Diarrhea 8.3 6.3 Headache 6.5 5.5 Nausea 6.2 4.6 Vomiting 3.7 2.0 Insomnia 2.5 1.7 Constipation 2.2 2.1 Rash 2.0 2.2 Dizziness 2.0 1.9 Fever 1.6 2.1 Other adverse events reported in Phase 2 and Phase 3 studies included oral moniliasis, vaginal moniliasis, hypertension, dyspepsia, localized abdominal pain, pruritus, and tongue discoloration.
Table 7 shows the incidence of drug-related adverse events reported in at least 1% of adult patients in these trials by dose of ZYVOX.
Table 7. Incidence (%) of Drug-Related Adverse Events Occurring in >1% of Adult Patients Treated with ZYVOX in Comparator-Controlled Clinical Trials Uncomplicated Skin and Skin Structure Infections All Other Indications Adverse Event ZYVOX 400 mg PO q12h
(n=548)Clarithromycin 250 mg PO q12h
(n=537)ZYVOX 600 mg q12h
(n=1498)All Other Comparators*
(n=1464)- *
- Comparators included cefpodoxime proxetil 200 mg PO q12h; ceftriaxone 1 g IV q12h; dicloxacillin 500 mg PO q6h; oxacillin 2 g IV q6h; vancomycin 1 g IV q12h.
- †
- The most commonly reported drug-related adverse events leading to discontinuation in patients treated with ZYVOX were nausea, headache, diarrhea, and vomiting.
% of patients with 1 drug-related adverse event 25.4 19.6 20.4 14.3 % of patients discontinuing due to drug-related adverse events† 3.5 2.4 2.1 1.7 Diarrhea 5.3 4.8 4.0 2.7 Nausea 3.5 3.5 3.3 1.8 Headache 2.7 2.2 1.9 1.0 Taste alteration 1.8 2.0 0.9 0.2 Vaginal moniliasis 1.6 1.3 1.0 0.4 Fungal infection 1.5 0.2 0.1 <0.1 Abnormal liver function tests 0.4 0 1.3 0.5 Vomiting 0.9 0.4 1.2 0.4 Tongue discoloration 1.1 0 0.2 0 Dizziness 1.1 1.5 0.4 0.3 Oral moniliasis 0.4 0 1.1 0.4 Pediatric Patients
The safety of ZYVOX formulations was evaluated in 215 pediatric patients ranging in age from birth through 11 years, and in 248 pediatric patients aged 5 through 17 years (146 of these 248 were age 5 through 11 and 102 were age 12 to 17). These patients were enrolled in two Phase 3 comparator-controlled clinical trials and were treated for up to 28 days. In these studies, 83% and 99%, respectively, of the adverse events reported with ZYVOX were described as mild to moderate in intensity. In the study of hospitalized pediatric patients (birth through 11 years) with Gram-positive infections, who were randomized 2 to 1 (linezolid:vancomycin), mortality was 6.0% (13/215) in the linezolid arm and 3.0% (3/101) in the vancomycin arm. However, given the severe underlying illness in the patient population, no causality could be established. Table 8 shows the incidence of adverse events reported in at least 2% of pediatric patients treated with ZYVOX in these trials.
Table 8. Incidence (%) of Adverse Events Reported in ≥2% of Pediatric Patients Treated with ZYVOX in Comparator-Controlled Clinical Trials Uncomplicated Skin and Skin Structure Infections* All Other Indications† Event ZYVOX
(n=248)Cefadroxil
(n = 251)ZYVOX
(n = 215)Vancomycin
(n=101)- *
- Patients 5 through 11 years of age received ZYVOX 10 mg/kg PO q12h or cefadroxil 15 mg/kg PO q12h. Patients 12 years or older received ZYVOX 600 mg PO q12h or cefadroxil 500 mg PO q12h.
- †
- Patients from birth through 11 years of age received ZYVOX 10 mg/kg IV/PO q8h or vancomycin 10 to 15 mg/kg IV q6–24h, depending on age and renal clearance.
Fever 2.9 3.6 14.1 14.1 Diarrhea 7.8 8.0 10.8 12.1 Vomiting 2.9 6.4 9.4 9.1 Sepsis 0 0 8.0 7.1 Rash 1.6 1.2 7.0 15.2 Headache 6.5 4.0 0.9 0 Anemia 0 0 5.6 7.1 Thrombocytopenia 0 0 4.7 2.0 Upper respiratory infection 3.7 5.2 4.2 1.0 Nausea 3.7 3.2 1.9 0 Dyspnea 0 0 3.3 1.0 Reaction at site of injection or of vascular catheter 0 0 3.3 5.1 Trauma 3.3 4.8 2.8 2.0 Pharyngitis 2.9 1.6 0.5 1.0 Convulsion 0 0 2.8 2.0 Hypokalemia 0 0 2.8 3.0 Pneumonia 0 0 2.8 2.0 Thrombocythemia 0 0 2.8 2.0 Cough 2.4 4.0 0.9 0 Generalized abdominal pain 2.4 2.8 0.9 2.0 Localized abdominal pain 2.4 2.8 0.5 1.0 Apnea 0 0 2.3 2.0 Gastrointestinal bleeding 0 0 2.3 1.0 Generalized edema 0 0 2.3 1.0 Loose stools 1.6 0.8 2.3 3.0 Localized pain 2.0 1.6 0.9 0 Skin disorder 2.0 0 0.9 1.0 Table 9 shows the incidence of drug-related adverse events reported in more than 1% of pediatric patients (and more than 1 patient) in either treatment group in the comparator-controlled Phase 3 trials.
Table 9. Incidence (%) of Drug-related Adverse Events Occurring in >1% of Pediatric Patients (and >1 Patient) in Either Treatment Group in Comparator-Controlled Clinical Trials Event Uncomplicated Skin and Skin Structure Infections* All Other Indications† ZYVOX
(n=248)Cefadroxil
(n=251)ZYVOX
(n=215)Vancomycin
(n=101)- *
- Patients 5 through 11 years of age received ZYVOX 10 mg/kg PO q12h or cefadroxil 15 mg/kg PO q12h. Patients 12 years or older received ZYVOX 600 mg PO q12h or cefadroxil 500 mg PO q12h.
- †
- Patients from birth through 11 years of age received ZYVOX 10 mg/kg IV/PO q8h or vancomycin 10 to 15 mg/kg IV q6–24h, depending on age and renal clearance.
- ‡
- These reports were of 'red-man syndrome', which were coded as anaphylaxis.
% of patients with ≥1 drug-related adverse event 19.2 14.1 18.8 34.3 % of patients discontinuing due to a drug-related adverse event 1.6 2.4 0.9 6.1 Diarrhea 5.7 5.2 3.8 6.1 Nausea 3.3 2.0 1.4 0 Headache 2.4 0.8 0 0 Loose stools 1.2 0.8 1.9 0 Thrombocytopenia 0 0 1.9 0 Vomiting 1.2 2.4 1.9 1.0 Generalized abdominal pain 1.6 1.2 0 0 Localized abdominal pain 1.6 1.2 0 0 Anemia 0 0 1.4 1.0 Eosinophilia 0.4 0.4 1.4 0 Rash 0.4 1.2 1.4 7.1 Vertigo 1.2 0.4 0 0 Oral moniliasis 0 0 0.9 4.0 Fever 0 0 0.5 3.0 Pruritus at non-application site 0.4 0 0 2.0 Anaphylaxis 0 0 0 10.1‡ Laboratory Changes
ZYVOX has been associated with thrombocytopenia when used in doses up to and including 600 mg every 12 hours for up to 28 days. In Phase 3 comparator-controlled trials, the percentage of adult patients who developed a substantially low platelet count (defined as less than 75% of lower limit of normal and/or baseline) was 2.4% (range among studies: 0.3 to 10.0%) with ZYVOX and 1.5% (range among studies: 0.4 to 7.0%) with a comparator. In a study of hospitalized pediatric patients ranging in age from birth through 11 years, the percentage of patients who developed a substantially low platelet count (defined as less than 75% of lower limit of normal and/or baseline) was 12.9% with ZYVOX and 13.4% with vancomycin. In an outpatient study of pediatric patients aged from 5 through 17 years, the percentage of patients who developed a substantially low platelet count was 0% with ZYVOX and 0.4% with cefadroxil. Thrombocytopenia associated with the use of ZYVOX appears to be dependent on duration of therapy, (generally greater than 2 weeks of treatment). The platelet counts for most patients returned to the normal range/baseline during the follow-up period. No related clinical adverse events were identified in Phase 3 clinical trials in patients developing thrombocytopenia. Bleeding events were identified in thrombocytopenic patients in a compassionate use program for ZYVOX; the role of linezolid in these events cannot be determined (see WARNINGS).
Changes seen in other laboratory parameters, without regard to drug relationship, revealed no substantial differences between ZYVOX and the comparators. These changes were generally not clinically significant, did not lead to discontinuation of therapy, and were reversible. The incidence of adult and pediatric patients with at least one substantially abnormal hematologic or serum chemistry value is presented in Tables 10, 11, 12, and 13.
Table 10. Percent of Adult Patients who Experienced at Least One Substantially Abnormal* Hematology Laboratory Value in Comparator-Controlled Clinical Trials with ZYVOX Laboratory Assay Uncomplicated Skin and Skin Structure Infections All Other Indications ZYVOX
400 mg q12hClarithromycin
250 mg q12hZYVOX
600 mg q12hAll Other Comparators† - *
- <75% (<50% for neutrophils) of Lower Limit of Normal (LLN) for values normal at baseline;
<75% (<50% for neutrophils) of LLN and of baseline for values abnormal at baseline. - †
- Comparators included cefpodoxime proxetil 200 mg PO q12h; ceftriaxone 1 g IV q12h; dicloxacillin 500 mg PO q6h; oxacillin 2 g IV q6h; vancomycin 1 g IV q12h.
Hemoglobin (g/dL) 0.9 0.0 7.1 6.6 Platelet count (x 103/mm3) 0.7 0.8 3.0 1.8 WBC (x 103/mm3) 0.2 0.6 2.2 1.3 Neutrophils (x 103/mm3) 0.0 0.2 1.1 1.2 Table 11. Percent of Adult Patients who Experienced at Least One Substantially Abnormal* Serum Chemistry Laboratory Value in Comparator-Controlled Clinical Trials with ZYVOX Laboratory Assay Uncomplicated Skin and Skin Structure Infections All Other Indications ZYVOX
400 mg q12hClarithromycin
250 mg q12hZYVOX
600 mg q12hAll Other
Comparators†AST (U/L) 1.7 1.3 5.0 6.8 ALT (U/L) 1.7 1.7 9.6 9.3 LDH (U/L) 0.2 0.2 1.8 1.5 Alkaline phosphatase (U/L) 0.2 0.2 3.5 3.1 Lipase (U/L) 2.8 2.6 4.3 4.2 Amylase (U/L) 0.2 0.2 2.4 2.0 Total bilirubin (mg/dL) 0.2 0.0 0.9 1.1 BUN (mg/dL) 0.2 0.0 2.1 1.5 Creatinine (mg/dL) 0.2 0.0 0.2 0.6 Table 12. Percent of Pediatric Patients who Experienced at Least One Substantially Abnormal* Hematology Laboratory Value in Comparator-Controlled Clinical Trials with ZYVOX Laboratory Assay Uncomplicated Skin and Skin Structure Infections† All Other Indications‡ ZYVOX Cefadroxil ZYVOX Vancomycin - *
- <75% (<50% for neutrophils) of Lower Limit of Normal (LLN) for values normal at baseline;
<75% (<50% for neutrophils) of LLN and <75% (<50% for neutrophils, <90% for hemoglobin if baseline <LLN) of baseline for values abnormal at baseline. - †
- Patients 5 through 11 years of age received ZYVOX 10 mg/kg PO q12h or cefadroxil 15 mg/kg PO q12h. Patients 12 years or older received ZYVOX 600 mg PO q12h or cefadroxil 500 mg PO q12h.
- ‡
- Patients from birth through 11 years of age received ZYVOX 10 mg/kg IV/PO q8h or vancomycin 10 to 15 mg/kg IV q6–24h, depending on age and renal clearance.
Hemoglobin (g/dL) 0.0 0.0 15.7 12.4 Platelet count (x 103/mm3) 0.0 0.4 12.9 13.4 WBC (x 103/mm3) 0.8 0.8 12.4 10.3 Neutrophils (x 103/mm3) 1.2 0.8 5.9 4.3 Table 13. Percent of Pediatric Patients who Experienced at Least One Substantially Abnormal* Serum Chemistry Laboratory Value in Comparator-Controlled Clinical Trials with ZYVOX Laboratory Assay Uncomplicated Skin and Skin Structure Infections† All Other Indications‡ ZYVOX Cefadroxil ZYVOX Vancomycin - *
- >2 x Upper Limit of Normal (ULN) for values normal at baseline; >2 x ULN and >2 (>1.5 for total bilirubin) x baseline for values abnormal at baseline.
- †
- Patients 5 through 11 years of age received ZYVOX 10 mg/kg PO q12h or cefadroxil 15 mg/kg PO q12h. Patients 12 years or older received ZYVOX 600 mg PO q12h or cefadroxil 500 mg PO q12h.
- ‡
- Patients from birth through 11 years of age received ZYVOX 10 mg/kg IV/PO q8h or vancomycin 10 to 15 mg/kg IV q6–24h, depending on age and renal clearance.
ALT (U/L) 0.0 0.0 10.1 12.5 Lipase (U/L) 0.4 1.2 --- --- Amylase (U/L) --- --- 0.6 1.3 Total bilirubin (mg/dL) --- --- 6.3 5.2 Creatinine (mg/dL) 0.4 0.0 2.4 1.0 Postmarketing Experience
Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia) has been reported during postmarketing use of ZYVOX (see WARNINGS). Peripheral neuropathy, and optic neuropathy sometimes progressing to loss of vision, have been reported in patients treated with ZYVOX. Lactic acidosis has been reported with the use of ZYVOX (see PRECAUTIONS). Although these reports have primarily been in patients treated for longer than the maximum recommended duration of 28 days, these events have also been reported in patients receiving shorter courses of therapy. Serotonin syndrome has been reported in patients receiving concomitant serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and ZYVOX (see PRECAUTIONS). Convulsions have been reported with the use of ZYVOX (see PRECAUTIONS). Anaphylaxis, angioedema, and bullous skin disorders such as those described as Stevens Johnson syndrome have been reported. Superficial tooth discoloration and tongue discoloration have been reported with the use of linezolid. The tooth discoloration was removable with professional dental cleaning (manual descaling) in cases with known outcome. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to ZYVOX, or a combination of these factors. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made and causal relationship cannot be precisely established.
-
OVERDOSAGE
In the event of overdosage, supportive care is advised, with maintenance of glomerular filtration. Hemodialysis may facilitate more rapid elimination of linezolid. In a Phase 1 clinical trial, approximately 30% of a dose of linezolid was removed during a 3-hour hemodialysis session beginning 3 hours after the dose of linezolid was administered. Data are not available for removal of linezolid with peritoneal dialysis or hemoperfusion. Clinical signs of acute toxicity in animals were decreased activity and ataxia in rats and vomiting and tremors in dogs treated with 3000 mg/kg/day and 2000 mg/kg/day, respectively.
-
DOSAGE AND ADMINISTRATION
The recommended dosage for ZYVOX formulations for the treatment of infections is described in Table 14.
Table 14. Dosage Guidelines for ZYVOX
Infection*Dosage and Route of Administration Recommended Duration of Treatment (consecutive days) Pediatric Patients† (Birth through 11 Years of Age) Adults and Adolescents (12 Years and Older) - *
- Due to the designated pathogens (see INDICATIONS AND USAGE)
- †
- Neonates <7 days: Most pre-term neonates < 7 days of age (gestational age < 34 weeks) have lower systemic linezolid clearance values and larger AUC values than many full-term neonates and older infants. These neonates should be initiated with a dosing regimen of 10 mg/kg q12h. Consideration may be given to the use of 10 mg/kg q8h regimen in neonates with a sub-optimal clinical response. All neonatal patients should receive 10 mg/kg q8h by 7 days of life (see CLINICAL PHARMACOLOGY, Special Populations, Pediatric).
- ‡
- Oral dosing using either ZYVOX Tablets or ZYVOX for Oral Suspension
Complicated skin and skin structure infections 10 mg/kg IV or oral‡ q8h 600 mg IV or oral‡ q12h 10 to 14 Community-acquired pneumonia, including concurrent bacteremia Nosocomial pneumonia Vancomycin-resistant Enterococcus faecium infections, including concurrent bacteremia 10 mg/kg IV or oral‡ q8h 600 mg IV or oral‡ q12h 14 to 28 Uncomplicated skin and skin structure infections <5 yrs: 10 mg/kg oral‡ q8h
5–11 yrs: 10 mg/kg oral‡ q12hAdults: 400 mg oral‡ q12h
Adolescents: 600 mg oral‡ q12h10 to 14 Adult patients with infection due to MRSA should be treated with ZYVOX 600 mg q12h.
In limited clinical experience, 5 out of 6 (83%) pediatric patients with infections due to Gram-positive pathogens with MICs of 4 µg/mL treated with ZYVOX had clinical cures. However, pediatric patients exhibit wider variability in linezolid clearance and systemic exposure (AUC) compared with adults. In pediatric patients with a sub-optimal clinical response, particularly those with pathogens with MIC of 4 µg/mL, lower systemic exposure, site and severity of infection, and the underlying medical condition should be considered when assessing clinical response (see CLINICAL PHARMACOLOGY, Special Populations, Pediatric and PRECAUTIONS, Pediatric Use).
In controlled clinical trials, the protocol-defined duration of treatment for all infections ranged from 7 to 28 days. Total treatment duration was determined by the treating physician based on site and severity of the infection, and on the patient's clinical response.
No dose adjustment is necessary when switching from intravenous to oral administration. Patients whose therapy is started with ZYVOX I.V. Injection may be switched to either ZYVOX Tablets or Oral Suspension at the discretion of the physician, when clinically indicated.
Intravenous Administration
ZYVOX I.V. Injection is supplied in single-use, ready-to-use infusion bags (see HOW SUPPLIED for container sizes). Parenteral drug products should be inspected visually for particulate matter prior to administration. Check for minute leaks by firmly squeezing the bag. If leaks are detected, discard the solution, as sterility may be impaired.
ZYVOX I.V. Injection should be administered by intravenous infusion over a period of 30 to 120 minutes. Do not use this intravenous infusion bag in series connections. Additives should not be introduced into this solution. If ZYVOX I.V. Injection is to be given concomitantly with another drug, each drug should be given separately in accordance with the recommended dosage and route of administration for each product. In particular, physical incompatibilities resulted when ZYVOX I.V. Injection was combined with the following drugs during simulated Y-site administration: amphotericin B, chlorpromazine HCl, diazepam, pentamidine isothionate, erythromycin lactobionate, phenytoin sodium, and trimethoprim-sulfamethoxazole. Additionally, chemical incompatibility resulted when ZYVOX I.V. Injection was combined with ceftriaxone sodium.
If the same intravenous line is used for sequential infusion of several drugs, the line should be flushed before and after infusion of ZYVOX I.V. Injection with an infusion solution compatible with ZYVOX I.V. Injection and with any other drug(s) administered via this common line (see Compatible Intravenous Solutions).
Compatible Intravenous Solutions
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP
- Lactated Ringer's Injection, USP
Keep the infusion bags in the overwrap until ready to use. Store at room temperature. Protect from freezing. ZYVOX I.V. Injection may exhibit a yellow color that can intensify over time without adversely affecting potency.
Constitution of Oral Suspension
ZYVOX for Oral Suspension is supplied as a powder/granule for constitution. Gently tap bottle to loosen powder. Add a total of 123 mL distilled water in two portions. After adding the first half, shake vigorously to wet all of the powder. Then add the second half of the water and shake vigorously to obtain a uniform suspension. After constitution, each 5 mL of the suspension contains 100 mg of linezolid. Before using, gently mix by inverting the bottle 3 to 5 times. DO NOT SHAKE. Store constituted suspension at room temperature. Use within 21 days after constitution.
-
HOW SUPPLIED
Tablets
ZYVOX Tablets are available as follows:
600 mg (white, capsule-shaped, film-coated tablets printed with "ZYVOX 600 mg")
20 tablets in HDPE bottle NDC 21695-399-20 Storage of ZYVOX Formulations
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from light. Keep bottles tightly closed to protect from moisture. It is recommended that the infusion bags be kept in the overwrap until ready to use. Protect infusion bags from freezing.
-
CLINICAL STUDIES
Adults
Vancomycin-Resistant Enterococcal Infections
Adult patients with documented or suspected vancomycin-resistant enterococcal infection were enrolled in a randomized, multi-center, double-blind trial comparing a high dose of ZYVOX (600 mg) with a low dose of ZYVOX (200 mg) given every 12 hours (q12h) either intravenously (IV) or orally for 7 to 28 days. Patients could receive concomitant aztreonam or aminoglycosides. There were 79 patients randomized to high-dose linezolid and 66 to low-dose linezolid. The intent-to-treat (ITT) population with documented vancomycin-resistant enterococcal infection at baseline consisted of 65 patients in the high-dose arm and 52 in the low-dose arm.
The cure rates for the ITT population with documented vancomycin-resistant enterococcal infection at baseline are presented in Table 15 by source of infection. These cure rates do not include patients with missing or indeterminate outcomes. The cure rate was higher in the high-dose arm than in the low-dose arm, although the difference was not statistically significant at the 0.05 level.
Table 15. Cure Rates at the Test-of-Cure Visit for ITT Adult Patients with Documented Vancomycin-Resistant Enterococcal Infections at Baseline Source of Infection Cured ZYVOX
600 mg q12h
n/N (%)ZYVOX
200 mg q12h
n/N (%)- *
- Includes sources of infection such as hepatic abscess, biliary sepsis, necrotic gall bladder, pericolonic abscess, pancreatitis, and catheter-related infection.
Any site 39/58 (67) 24/46 (52) Any site with associated bacteremia 10/17 (59) 4/14 (29) Bacteremia of unknown origin 5/10 (50) 2/7 (29) Skin and skin structure 9/13 (69) 5/5 (100) Urinary tract 12/19 (63) 12/20 (60) Pneumonia 2/3 (67) 0/1 (0) Other* 11/13 (85) 5/13 (39) Nosocomial Pneumonia
Adult patients with clinically and radiologically documented nosocomial pneumonia were enrolled in a randomized, multi-center, double-blind trial. Patients were treated for 7 to 21 days. One group received ZYVOX I.V. Injection 600 mg q12h, and the other group received vancomycin 1 g q12h IV. Both groups received concomitant aztreonam (1 to 2 g every 8 hours IV), which could be continued if clinically indicated. There were 203 linezolid-treated and 193 vancomycin-treated patients enrolled in the study. One hundred twenty-two (60%) linezolid-treated patients and 103 (53%) vancomycin-treated patients were clinically evaluable. The cure rates in clinically evaluable patients were 57% for linezolid-treated patients and 60% for vancomycin-treated patients. The cure rates in clinically evaluable patients with ventilator-associated pneumonia were 47% for linezolid-treated patients and 40% for vancomycin-treated patients. A modified intent-to-treat (MITT) analysis of 94 linezolid-treated patients and 83 vancomycin-treated patients included subjects who had a pathogen isolated before treatment. The cure rates in the MITT analysis were 57% in linezolid-treated patients and 46% in vancomycin-treated patients. The cure rates by pathogen for microbiologically evaluable patients are presented in Table 16.
Table 16. Cure Rates at the Test-of-Cure Visit for Microbiologically Evaluable Adult Patients with Nosocomial Pneumonia Pathogen Cured ZYVOX
n/N (%)Vancomycin
n/N (%)Staphylococcus aureus 23/38 (61) 14/23 (61) Methicillin-resistant S. aureus 13/22 (59) 7/10 (70) Streptococcus pneumoniae 9/9 (100) 9/10 (90) Pneumonia caused by multi-drug resistant S.pneumoniae (MDRSP3)
ZYVOX was studied for the treatment of community-acquired (CAP) and hospital-acquired (HAP) pneumonia due to MDRSP by pooling clinical data from seven comparative and non-comparative Phase 2 and Phase 3 studies involving adult and pediatric patients. The pooled MITT population consisted of all patients with S.pneumoniae isolated at baseline; the pooled ME population consisted of patients satisfying criteria for microbiologic evaluability. The pooled MITT population with CAP included 15 patients (41%) with severe illness (risk classes IV and V) as assessed by a prediction rule11. The pooled clinical cure rates for patients with CAP due to MDRSP were 35/48 (73%) in the MITT and 33/36 (92%) in the ME populations respectively. The pooled clinical cure rates for patients with HAP due to MDRSP were 12/18 (67%) in the MITT and 10/12 (83%) in the ME populations respectively.
Table 17. Clinical cure rates for 36 microbiologically-evaluable patients with CAP due to MDRSP who were treated with ZYVOX (stratified by antibiotic susceptibility) Susceptibility Screening Clinical Cure n/N* (%) Penicillin-resistant 14/16 88 2nd generation cephalosporin-resistant† 19/22 86 Macrolide-resistant‡ 29/30 97 Tetracycline-resistant 22/24 92 Trimethoprim/sulfamethoxazole-resistant 18/21 86
- 3
- MDRSP refers to isolates resistant to two or more of the following antibiotics: penicillin, second-generation cephalosporins, macrolides, tetracycline, and trimethoprim/sulfamethoxazole.
Complicated Skin and Skin Structure Infections
Adult patients with clinically documented complicated skin and skin structure infections were enrolled in a randomized, multi-center, double-blind, double-dummy trial comparing study medications administered IV followed by medications given orally for a total of 10 to 21 days of treatment. One group of patients received ZYVOX I.V. Injection 600 mg q12h followed by ZYVOX Tablets 600 mg q12h; the other group received oxacillin 2 g every 6 hours (q6h) IV followed by dicloxacillin 500 mg q6h orally. Patients could receive concomitant aztreonam if clinically indicated. There were 400 linezolid-treated and 419 oxacillin-treated patients enrolled in the study. Two hundred forty-five (61%) linezolid-treated patients and 242 (58%) oxacillin-treated patients were clinically evaluable. The cure rates in clinically evaluable patients were 90% in linezolid-treated patients and 85% in oxacillin-treated patients. A modified intent-to-treat (MITT) analysis of 316 linezolid-treated patients and 313 oxacillin-treated patients included subjects who met all criteria for study entry. The cure rates in the MITT analysis were 86% in linezolid-treated patients and 82% in oxacillin-treated patients. The cure rates by pathogen for microbiologically evaluable patients are presented in Table 18.
Table 18. Cure Rates at the Test-of-Cure Visit for Microbiologically Evaluable Adult Patients with Complicated Skin and Skin Structure Infections Pathogen Cured ZYVOX
n/N (%)Oxacillin/Dicloxacillin
n/N (%)Staphylococcus aureus 73/83 (88) 72/84 (86) Methicillin-resistant S. aureus 2/3 (67) 0/0 (-) Streptococcus agalactiae 6/6 (100) 3/6 (50) Streptococcus pyogenes 18/26 (69) 21/28 (75) A separate study provided additional experience with the use of ZYVOX in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. This was a randomized, open-label trial in hospitalized adult patients with documented or suspected MRSA infection.
One group of patients received ZYVOX I.V. Injection 600 mg q12h followed by ZYVOX Tablets 600 mg q12h. The other group of patients received vancomycin 1 g q12h IV. Both groups were treated for 7 to 28 days, and could receive concomitant aztreonam or gentamicin if clinically indicated. The cure rates in microbiologically evaluable patients with MRSA skin and skin structure infection were 26/33 (79%) for linezolid-treated patients and 24/33 (73%) for vancomycin-treated patients.
Diabetic Foot Infections
Adult diabetic patients with clinically documented complicated skin and skin structure infections ("diabetic foot infections") were enrolled in a randomized (2:1 ratio), multi-center, open-label trial comparing study medications administered IV or orally for a total of 14 to 28 days of treatment. One group of patients received ZYVOX 600 mg q12h IV or orally; the other group received ampicillin/sulbactam 1.5 to 3 g IV or amoxicillin/clavulanate 500 to 875 mg every 8 to 12 hours (q8–12h) orally. In countries where ampicillin/sulbactam is not marketed, amoxicillin/clavulanate 500 mg to 2 g every 6 hours (q6h) was used for the intravenous regimen. Patients in the comparator group could also be treated with vancomycin 1 g q12h IV if MRSA was isolated from the foot infection. Patients in either treatment group who had Gram-negative bacilli isolated from the infection site could also receive aztreonam 1 to 2 g q8–12h IV. All patients were eligible to receive appropriate adjunctive treatment methods, such as debridement and off-loading, as typically required in the treatment of diabetic foot infections, and most patients received these treatments. There were 241 linezolid-treated and 120 comparator-treated patients in the intent-to-treat (ITT) study population. Two hundred twelve (86%) linezolid-treated patients and 105 (85%) comparator-treated patients were clinically evaluable. In the ITT population, the cure rates were 68.5% (165/241) in linezolid-treated patients and 64% (77/120) in comparator-treated patients, where those with indeterminate and missing outcomes were considered failures. The cure rates in the clinically evaluable patients (excluding those with indeterminate and missing outcomes) were 83% (159/192) and 73% (74/101) in the linezolid- and comparator-treated patients, respectively. A critical post-hoc analysis focused on 121 linezolid-treated and 60 comparator-treated patients who had a Gram-positive pathogen isolated from the site of infection or from blood, who had less evidence of underlying osteomyelitis than the overall study population, and who did not receive prohibited antimicrobials. Based upon that analysis, the cure rates were 71% (86/121) in the linezolid-treated patients and 63% (38/60) in the comparator-treated patients. None of the above analyses were adjusted for the use of adjunctive therapies. The cure rates by pathogen for microbiologically evaluable patients are presented in Table 19.
Table 19. Cure Rates at the Test-of-Cure Visit for Microbiologically Evaluable Adult Patients with Diabetic Foot Infections Pathogen Cured ZYVOX
n/N (%)Comparator
n/N (%)Staphylococcus aureus 49/63 (78) 20/29 (69) Methicillin-resistant S. aureus 12/17 (71) 2/3 (67) Streptococcus agalactiae 25/29 (86) 9/16 (56) Pediatric Patients
Infections Due to Gram-positive Organisms
A safety and efficacy study provided experience on the use of ZYVOX in pediatric patients for the treatment of nosocomial pneumonia, complicated skin and skin structure infections, catheter-related bacteremia, bacteremia of unidentified source, and other infections due to Gram-positive bacterial pathogens, including methicillin-resistant and -susceptible Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Pediatric patients ranging in age from birth through 11 years with infections caused by the documented or suspected Gram-positive organisms were enrolled in a randomized, open-label, comparator-controlled trial. One group of patients received ZYVOX I.V. Injection 10 mg/kg every 8 hours (q8h) followed by ZYVOX for Oral Suspension 10 mg/kg q8h. A second group received vancomycin 10 to 15 mg/kg IV every 6 to 24 hours, depending on age and renal clearance. Patients who had confirmed VRE infections were placed in a third arm of the study and received ZYVOX 10 mg/kg q8h IV and/or orally. All patients were treated for a total of 10 to 28 days and could receive concomitant Gram-negative antibiotics if clinically indicated. In the intent-to-treat (ITT) population, there were 206 patients randomized to linezolid and 102 patients randomized to vancomycin. One hundred seventeen (57 %) linezolid-treated patients and 55 (54%) vancomycin-treated patients were clinically evaluable. The cure rates in ITT patients were 81% in patients randomized to linezolid and 83% in patients randomized to vancomycin (95% Confidence Interval of the treatment difference; -13%, 8%). The cure rates in clinically evaluable patients were 91% in linezolid-treated patients and 91% in vancomycin-treated patients (95% CI; -11%, 11%). Modified intent-to-treat (MITT) patients included ITT patients who, at baseline, had a Gram-positive pathogen isolated from the site of infection or from blood. The cure rates in MITT patients were 80% in patients randomized to linezolid and 90% in patients randomized to vancomycin (95% CI; -23%, 3%). The cure rates for ITT, MITT, and clinically evaluable patients are presented in Table 20. After the study was completed, 13 additional patients ranging from 4 days through 16 years of age were enrolled in an open-label extension of the VRE arm of the study. Table 21 provides clinical cure rates by pathogen for microbiologically evaluable patients including microbiologically evaluable patients with vancomycin-resistant Enterococcus faecium from the extension of this study.
Table 20. Cure Rates at the Test-of-Cure Visit for Intent to Treat, Modified Intent to Treat, and Clinically Evaluable Pediatric Patients by Baseline Diagnosis Population ITT MITT* Clinically Evaluable ZYVOX
n/N (%)Vancomycin
n/N (%)ZYVOX
n/N (%)Vancomycin
n/N (%)ZYVOX
n/N (%)Vancomycin
n/N (%)- *
- MITT = ITT patients with an isolated Gram-positive pathogen at baseline
Any diagnosis 150/186 (81) 69/83 (83) 86/108 (80) 44/49 (90) 106/117 (91) 49/54 (91) Bacteremia of unidentified source 22/29 (76) 11/16 (69) 8/12 (67) 7/8 (88) 14/17 (82) 7/9 (78) Catheter-related bacteremia 30/41 (73) 8/12 (67) 25/35 (71) 7/10 (70) 21/25(84) 7/9 (78) Complicated skin and skin structure infections 61/72 (85) 31/34 (91) 37/43 (86) 22/23 (96) 46/49 (94) 26/27 (96) Nosocomial pneumonia 13/18 (72) 11/12 (92) 5/6 (83) 4/4 (100) 7/7 (100) 5/5 (100) Other infections 24/26 (92) 8/9 (89) 11/12 (92) 4/4 (100) 18/19 (95) 4/4 (100) Table 21. Cure Rates at the Test-of-Cure Visit for Microbiologically Evaluable Pediatric Patients with Infections due to Gram-positive Pathogens Pathogen Microbiologically Evaluable ZYVOX
n/N (%)Vancomycin
n/N (%)- *
- Includes data from 7 patients enrolled in the open-label extension of this study.
Vancomycin-resistant Enterococcus faecium 6/8 (75)* 0/0 (-) Staphylococcus aureus 36/38 (95) 23/24 (96) Methicillin-resistant S. aureus 16/17 (94) 9/9 (100) Streptococcus pyogenes 2/2 (100) 1/2 (50) -
REFERENCES
- Gonzales RD, PC Schreckenberger, MB Graham, et al. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. The Lancet 2001;357:1179.
- Herrero IA, NC Issa, R Patel. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. The New England Journal of Medicine 2002;346:867–869.
- Tsiodras S, HS Gold, G Sakoulas, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus. The Lancet 2001;358:207–208.
- Goldman DA, RA Weinstein, RP Wenzel, et al. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. The Journal of the American Medical Association 1996;275:234–240.
- Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morbidity and Mortality Weekly Report 2002;51 (RR-16).
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol. 20, No. 2, NCCLS, Wayne, PA, January 2000.
- National Committee for Clinical Laboratory Standards. Twelfth Informational Supplement. Approved NCCLS Document M100-S12, Vol. 21, No. 1, NCCLS, Wayne, PA, January 2002.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. Seventh Edition. Approved Standard NCCLS Document M2-A7, Vol. 20, No. 1, NCCLS, Wayne, PA, January 2000.
- Walker SE et al. Tyramine content of previously restricted foods in monoamine oxidase inhibitor diets. Journal of Clinical Psychopharmacology 1996;16(5):383–388.
- DaPrada M et al. On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. Journal of Neural Transmission 1988; [Supplement] 26:31–56.
- Fine MJ, Auble TE, Yealy DM, et al. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. The New England Journal of Medicine. 1997;336 (4):243–250.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ZYVOX
linezolid tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-399(NDC:0009-5135) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength linezolid (UNII: ISQ9I6J12J) (linezolid - UNII:ISQ9I6J12J) linezolid 600 mg Inactive Ingredients Ingredient Name Strength starch, corn (UNII: O8232NY3SJ) cellulose, microcrystalline (UNII: OP1R32D61U) hydroxypropyl cellulose (UNII: RFW2ET671P) magnesium stearate (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) polyethylene glycol (UNII: 3WJQ0SDW1A) titanium dioxide (UNII: 15FIX9V2JP) carnauba wax (UNII: R12CBM0EIZ) Product Characteristics Color WHITE Score no score Shape OVAL (CAPSULE-SHAPED) Size 18mm Flavor Imprint Code ZYVOX;600mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-399-20 20 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021130 04/18/2000 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK