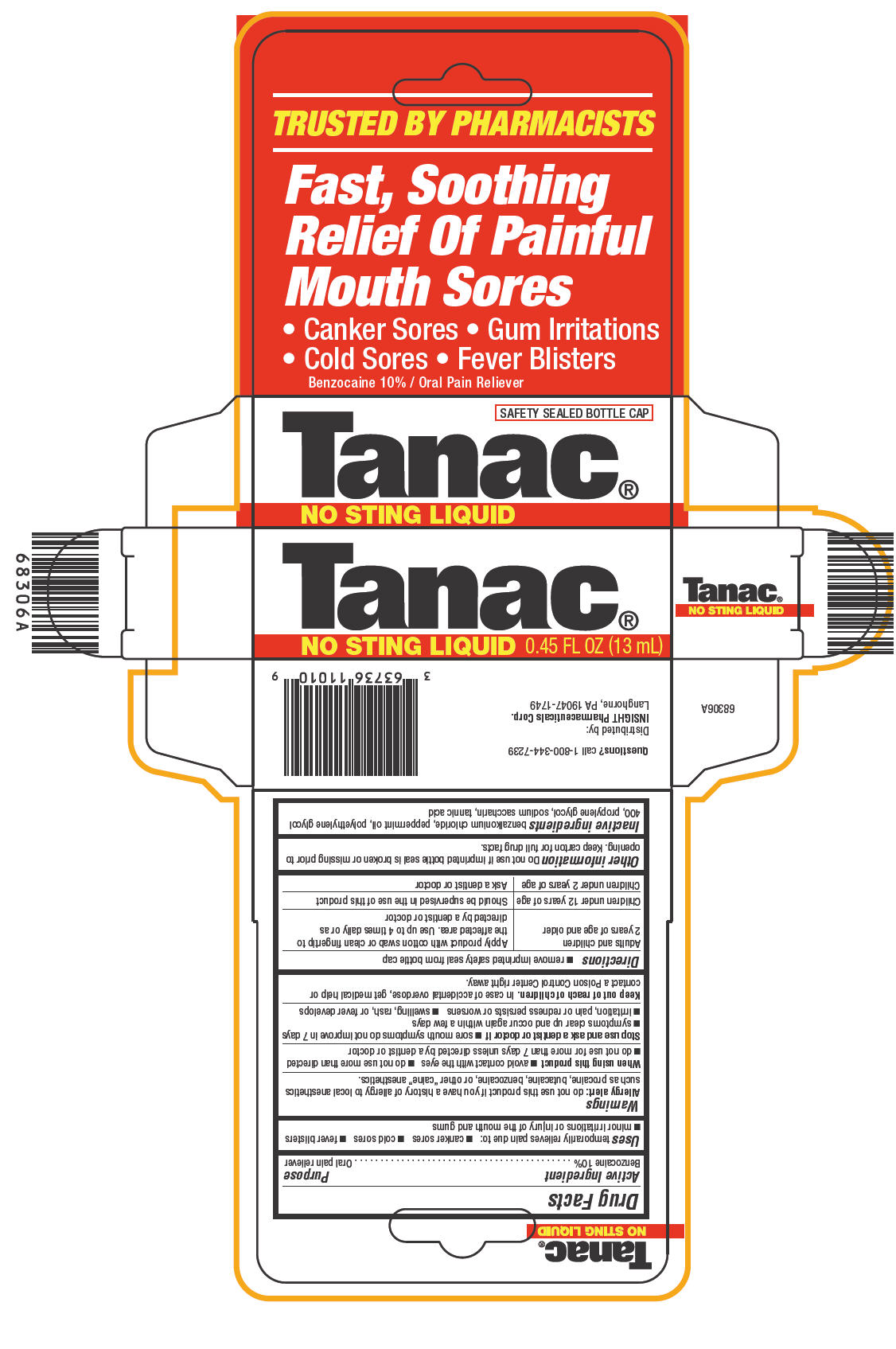
TANAC- benzocaine liquid
Insight Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Tanac®
NO STING LIQUID
Uses
temporarily relieves pain due to:
- canker sores
- cold sores
- fever blisters
- minor irritations or injury of the mouth and gums
Warnings
Allergy alert
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- avoid contact with the eyes
- do not use more than directed
- do not use for more than 7 days unless directed by a dentist or doctor
Directions
- remove imprinted safety seal from bottle cap
| Adults and children 2 years of age and older | Apply product with cotton swab or clean fingertip to the affected area. Use up to 4 times daily or as directed by a dentist or doctor |
| Children under 12 years of age | Should be supervised in the use of this product |
| Children under 2 years of age | Ask a dentist or doctor |
Other information
Do not use if imprinted bottle seal is broken or missing prior to opening. Keep carton for full drug facts.
| TANAC
benzocaine liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Insight Pharmaceuticals LLC (055665422) |
Revised: 7/2010
Document Id: f8999233-536d-4588-a7a6-42681fd078af
Set id: 4fec813b-f5ad-4d15-8db5-529d5aa8bcb6
Version: 2
Effective Time: 20100729
Insight Pharmaceuticals LLC