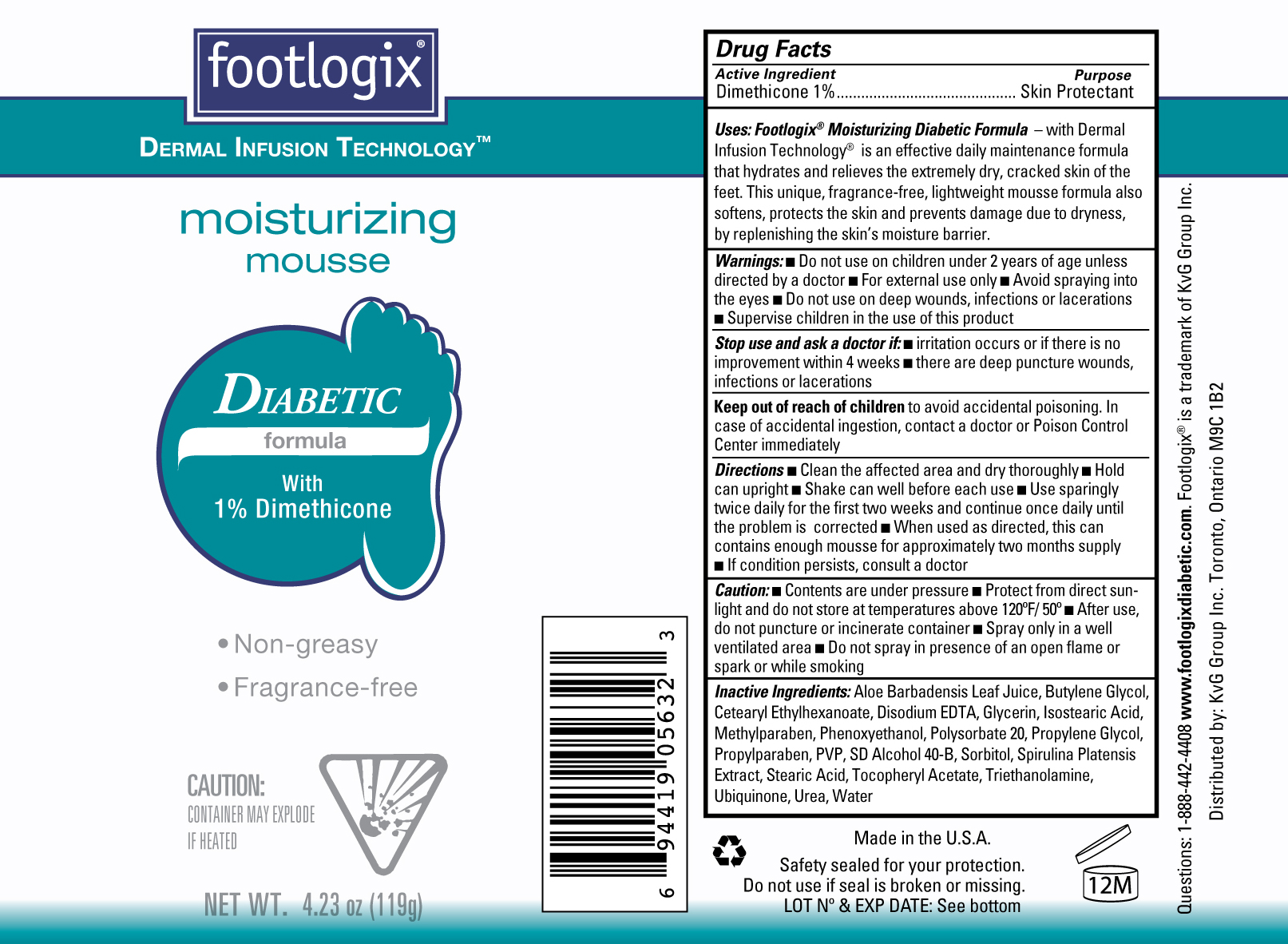
Label: FOOTLOGIX MOISTURIZING DIABETIC FORMULA- dimethicone aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 42479-214-04 - Packager: KVG Group Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 28, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses: Footlogix Moisturizing Diabetic Formula- with Dermal Infusion Technology is an effective daily maintenance formula that hydrates and relieves the extremely dry, cracked skin of the feet. This unique fragrance-free, lightweight mousse formula also softens, protects the skni and prevents damage due to dryness by replenishing the skin's moisture barrier.
- WARNINGS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions ■ Clean the affected area and dry thoroughly ■ Hold the can upright ■ Shake well before each use ■ Use sparingly twice daily for the first two weeks and continue once daily until the problem is corrected ■ When used as directed, this can contains enough mousse for approximately two months supply ■ If condition persists, consult a doctor
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive Ingredients Aloe Barbadensis Leaf Juice, Butylene Glycol, Cetearyl Ethylhexanoate, Disodium EDTA, Glycerin, Isostearic Acid, Methylparaben, Phenoxyethanol, Polysorbate 20, Propylene Glycol, Propylparaben, PVP, SD Alcohol 40-B, Sorbitol, Spirulina Platensis Extract, Stearic Acid, Tocopheryl Acetate, Triethanolamine, Ubiquinone, Urea, Water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOOTLOGIX MOISTURIZING DIABETIC FORMULA
dimethicone aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42479-214 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ISOSTEARIC ACID (UNII: X33R8U0062) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) POVIDONE (UNII: FZ989GH94E) SORBITOL (UNII: 506T60A25R) SPIRULINA PLATENSIS (UNII: 9L3TIH1UUE) STEARIC ACID (UNII: 4ELV7Z65AP) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42479-214-04 119 g in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/15/2012 Labeler - KVG Group Inc (206932605)