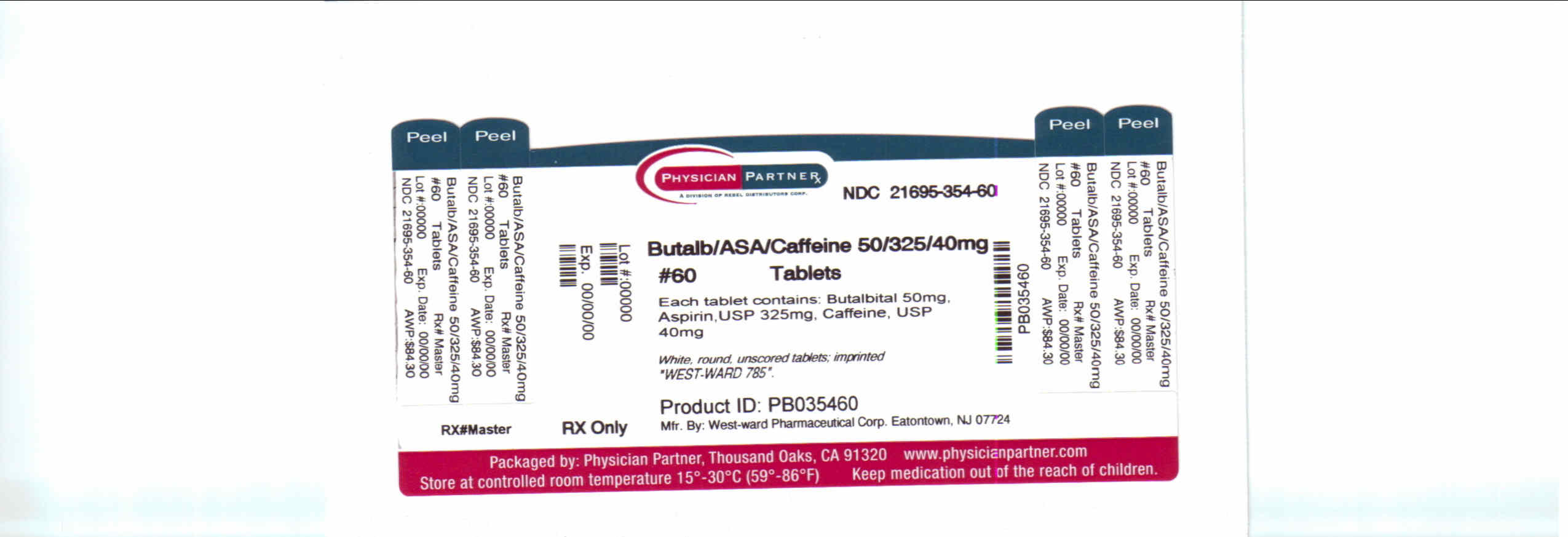
Label: BUTALBITAL, ASPIRIN AND CAFFEINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-354-60 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0143-1785
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 10, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Each Butalbital, Aspirin, and Caffeine Tablet for oral administration contains:
Butalbital, USP.....................50 mg
Aspirin, USP.......................325 mg
Caffeine, USP.......................40 mg
Butalbital, 5-allyl-5-isobutylbarbituric acid, a white, odorless, crystalline powder having a slightly bitter taste, is a short to intermediate-acting barbiturate. It has the following structural formula:
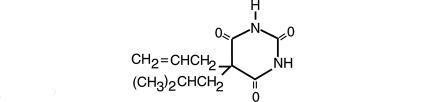
C11H16N2O3 M.W. 224.26
Aspirin, salicylic acid acetate, is a non-opiate analgesic, anti-inflammatory and antipyretic agent. It occurs as a white, crystalline tabular or needle-like powder and is odorless or has a faint odor. It has the following structural formula:
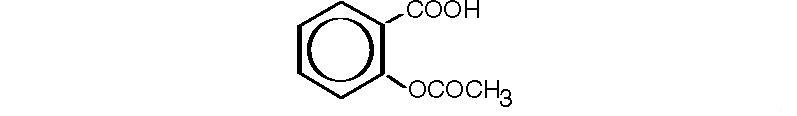
C9H8O4 M.W. 180.16
Caffeine, 1,3,7,-trimethylxanthine, is a central nervous system stimulant which occurs as a white powder or white glistening needles. It also has a bitter taste. Its structure is as follows:
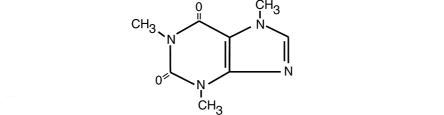
C8H10N4O2 M.W. 194.19
Inactive Ingredients: Corn Starch, Hydrogenated Vegetable Oil, Microcrystalline Cellulose, Pregelatinized Starch, Sodium Starch Glycolate and Talc.
-
CLINICAL PHARMACOLOGY:
Pharmacologically, Butalbital, Aspirin, and Caffeine Tablets combine the analgesic properties of aspirin with the anxiolytic and muscle relaxant properties of butalbital.
The clinical effectiveness of a product containing butalbital, aspirin, and caffeine in tension headache has been established in double-blind, placebo-controlled, multiclinic trials. A factorial design study compared the combination product with each of its major components. This study demonstrated that each component contributes to the efficacy of the combination product in the treatment of the target symptoms of tension headache (headache pain, psychic tension, and muscle contraction in the head, neck, and shoulder region). For each symptom and the symptom complex as a whole, the product containing butalbital, aspirin, and caffeine was shown to have significantly superior clinical effects to either of the major components alone.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
PRECAUTIONS:
1. General:
Butalbital, Aspirin, and Caffeine Tablets should be used with caution in patients with certain medical problems, including those with a history of asthma, allergies and nasal polyps. Also, the drug must be prescribed carefully for patients with hemophilia or other bleeding problems, peptic ulcer, renal impairment, or a history of drug abuse or dependence.
2. Information for Patients:
Butalbital, Aspirin, and Caffeine Tablets may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
3. Drug Interactions:
Patients receiving narcotic analgesics, antipsychotics, antianxiety agents, or other CNS depressants (including alcohol) concomitantly with Butalbital, Aspirin, and Caffeine Tablets may exhibit additive CNS depressant effects. When combined therapy is contemplated, the dose of one or both agents should be reduced.
DRUGS EFFECT Aspirin w/anti-inflammatory agents Increased
ulcerogenic effects.Butalbital w/coumarin anticoagulants Decreased effect of
anticoagulant
because of
increased
metabolism
resulting from
enzyme induction.Butalbital w/tricyclic antidepressants Decreased blood
levels of the
antidepressant.4. Usage in Pregnancy:
Adequate studies have not been performed in animals to determine whether this drug affects fertility in males or females, has teratogenic potential or has other adverse effects on the fetus. While there are no well-controlled studies in pregnant women, over twenty years of marketing and clinical experience does not include any positive evidence of adverse effects on the fetus. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Butalbital, Aspirin, and Caffeine Tablets should be used in pregnant women only when clearly needed.
-
ADVERSE REACTIONS:
The most frequent adverse reactions are drowsiness and dizziness. Less frequent adverse reactions are lightheadedness and gastrointestinal disturbances including nausea, vomiting, and flatulence. Mental confusion or depression can occur due to intolerance or overdosage of butalbital.
Several cases of dermatological reactions including toxic epidermal necrolysis and erythema multiforme have been reported.
-
DRUG ABUSE AND DEPENDENCE:
Butalbital, Aspirin, and Caffeine Tablets are classified as a Schedule III controlled substance.
Prolonged use of barbiturates can produce drug dependence, characterized by psychic dependence, and less frequently, physical dependence and tolerance. The abuse liability of Butalbital, Aspirin, and Caffeine Tablets are similar to that of other barbiturate-containing drug combinations. Caution should be exercised when prescribing medication for patients with a known propensity for taking excessive quantities of drugs, which is not uncommon in patients with chronic tension headache.
-
OVERDOSAGE:
Symptoms: The toxic effects of acute overdosage of Butalbital, Aspirin, and Caffeine Tablets are attributable mainly to its barbiturate component, and, to a lesser extent, aspirin. Because toxic effects of caffeine occur in very high dosages only, the possibility of significant caffeine toxicity from Butalbital, Aspirin, and Caffeine Tablet overdosage in unlikely. Symptoms attributable to acute barbiturate poisoning include drowsiness, confusion, and coma; respiratory depression; hypotension; shock. Symptoms attributable to acute aspirin poisoning include hyperpnea; acid-base disturbances with development of metabolic acidosis; vomiting and abdominal pain; tinnitus; hyperthermia; hypoprothrombinemia; restlessness; delirium; convulsions. Acute caffeine poisoning may cause insomnia, restlessness, tremor, and delirium; tachycardia and extrasystoles.
Treatment: Treatment consists primarily of management of barbiturate intoxication and the correction of the acid-base imbalance due to salicylism. Vomiting should be induced mechanically or with emetics in the conscious patient. Gastric lavage may be used if the pharyngeal and laryngeal reflexes are present and if less than four hours have elapsed since ingestion. A cuffed endotracheal tube should be inserted before gastric lavage of the unconscious patient and when necessary to provide assisted respiration. Diuresis, alkalinization of the urine, and correction of electrolyte disturbances should be accomplished through administration of intravenous fluids such as 1% sodium bicarbonate in 5% dextrose injection. Meticulous attention should be given to maintaining adequate pulmonary ventilation. Correction of hypotension may require the administration of norepinephrine bitartrate of phenylephrine hydrochloride by intravenous infusion. In severe cases of intoxication, peritoneal dialysis, hemodialysis, or exchange transfusion may be lifesaving. Hypoprothrombinemia should be treated with Vitamin K, intravenously.
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
Butalbital, Aspirin and Caffeine Tablets, USP 50 mg/325 mg/40 mg: White, round, unscored tablets; imprinted “West-ward 785”.
Bottles of 60 tablets, NDC 21695-354-60.
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured By:
West-ward Pharmaceutical Corp.
Eatontown, NJ 07724
Revised April 2003Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BUTALBITAL, ASPIRIN AND CAFFEINE
butalbital, aspirin and caffeine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-354(NDC:0143-1785) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTALBITAL (UNII: KHS0AZ4JVK) (BUTALBITAL - UNII:KHS0AZ4JVK) BUTALBITAL 50 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 40 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code West;ward;785 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-354-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA086162 12/15/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK