Label: MINOXIDIL FOR WOMEN- minoxidil solution
- NDC Code(s): 68016-006-64
- Packager: Chain Drug Consortium, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
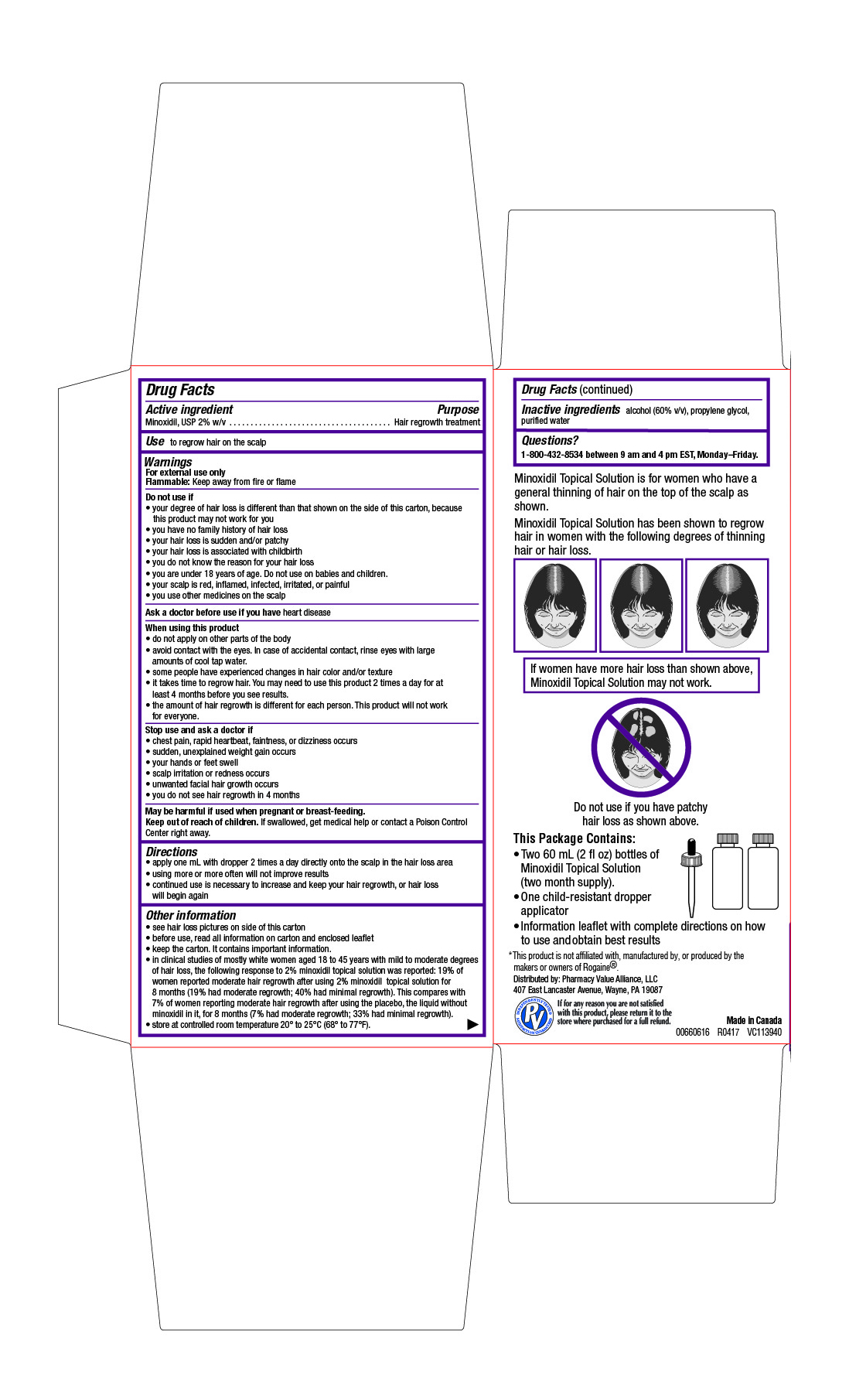
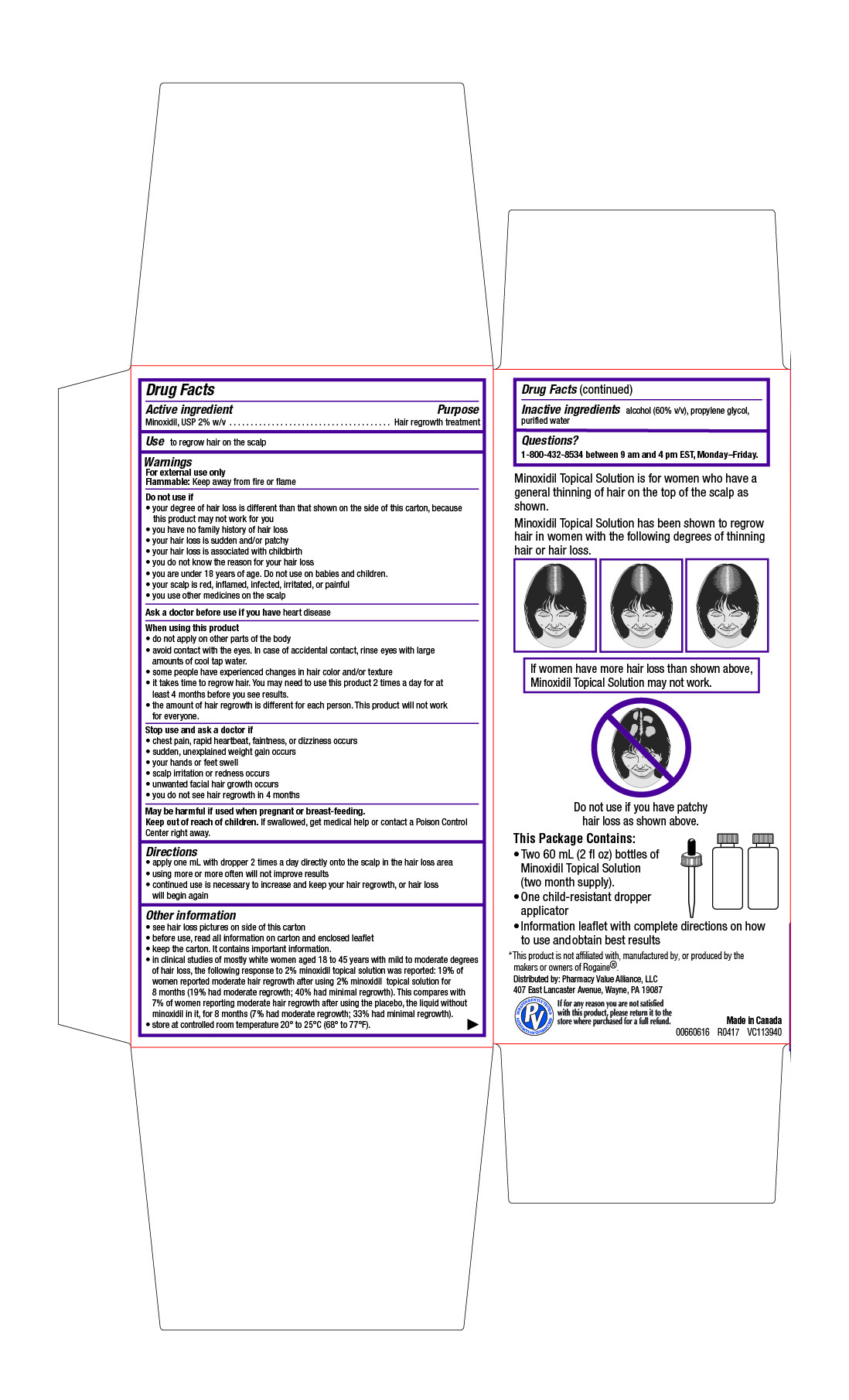
- Drug Facts
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Flammable: Keep away from fire or flameDo not use if
- your degree of hair loss is different than that shown on the side of this carton, because this product may not work for you
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- your hair loss is associated with childbirth
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medicines on the scalp
When using this product
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. You may need to use this product 2 times a day for at least 4 months before you see results.
- the amount of hair regrowth is different for each person. This product will not work for everyone.
- Directions
-
Other information
- see hair loss pictures on side of this carton
- before use, read all information on carton and enclosed leaflet
- keep the carton. It contains important information.
- in clinical studies of mostly white women aged 18 to 45 years with mild to moderate degrees of hair loss, the following response to 2% minoxidil topical solution was reported: 19% of women reported moderate hair regrowth after using 2% minoxidil topical solution for 8 months (19% had moderate regrowth; 40% had minimal regrowth). This compares with 7% of women reporting moderate hair regrowth after using the placebo, the liquid without minoxidil in it, for 8 months (7% had moderate regrowth; 33% had minimal regrowth).
- store at controlled room temperature 20° to 25°C (68° to 77°F).
- Inactive ingredients
- Questions?
-
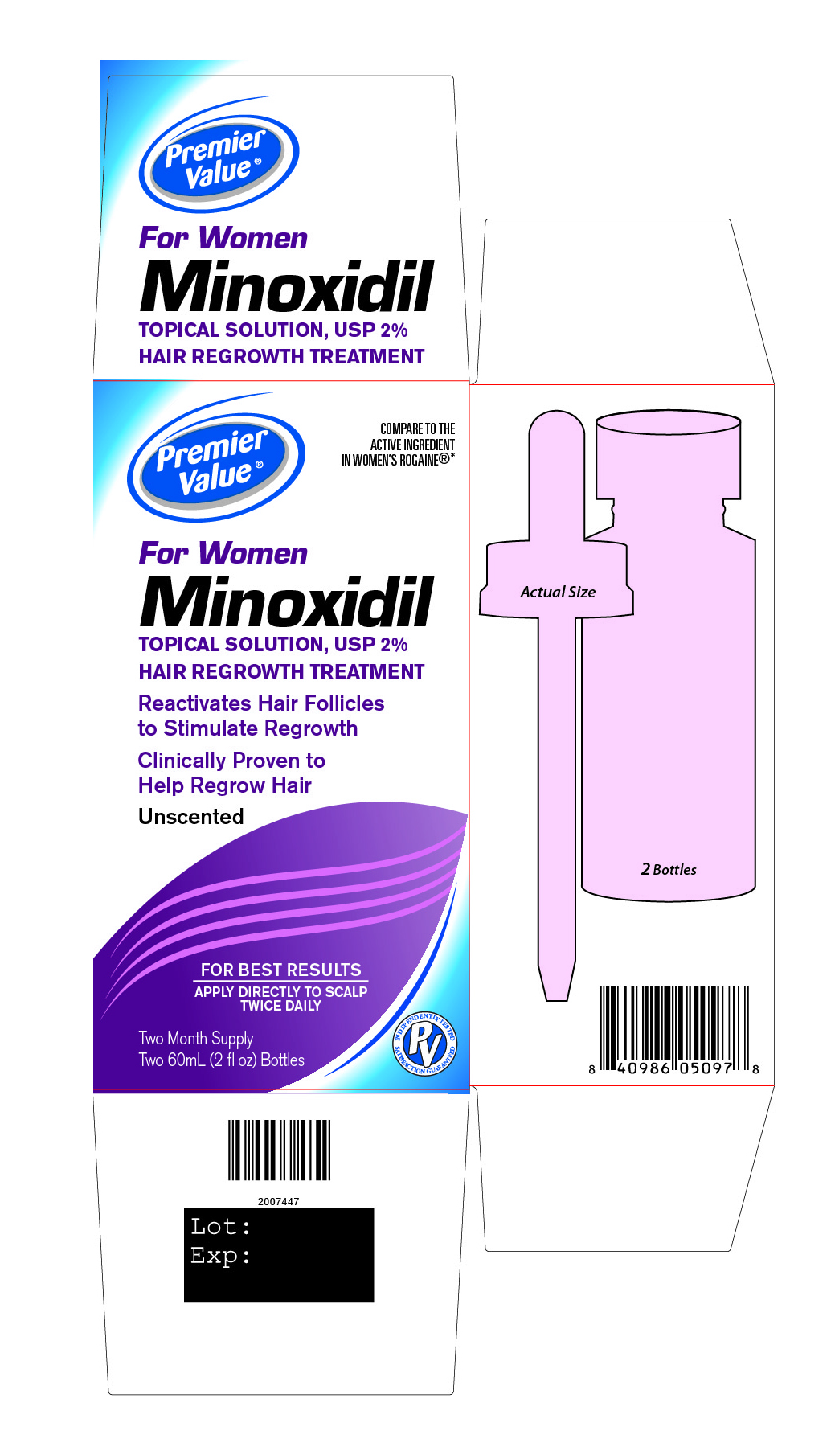
Principal Display Panel
Premier Value®
COMPARE TO THE ACTIVE INGREDIENT IN WOMEN’S ROGAINE®*
For Women
Minoxidil
TOPICAL SOLUTION, USP 2%
HAIR REGROWTH TREATMENTReactivates Hair Follicles
to stimulate regrowthClinically Proven to
Help Regrow HairUnscented
FOR BEST RESULTS
APPLY DIRECTLY TO SCALP
TWICE DAILYINDEPENDENTLY TESTED SATISFACTION GUARANTEED
Two Month Supply
Two 60 mL (2 fl oz) Bottles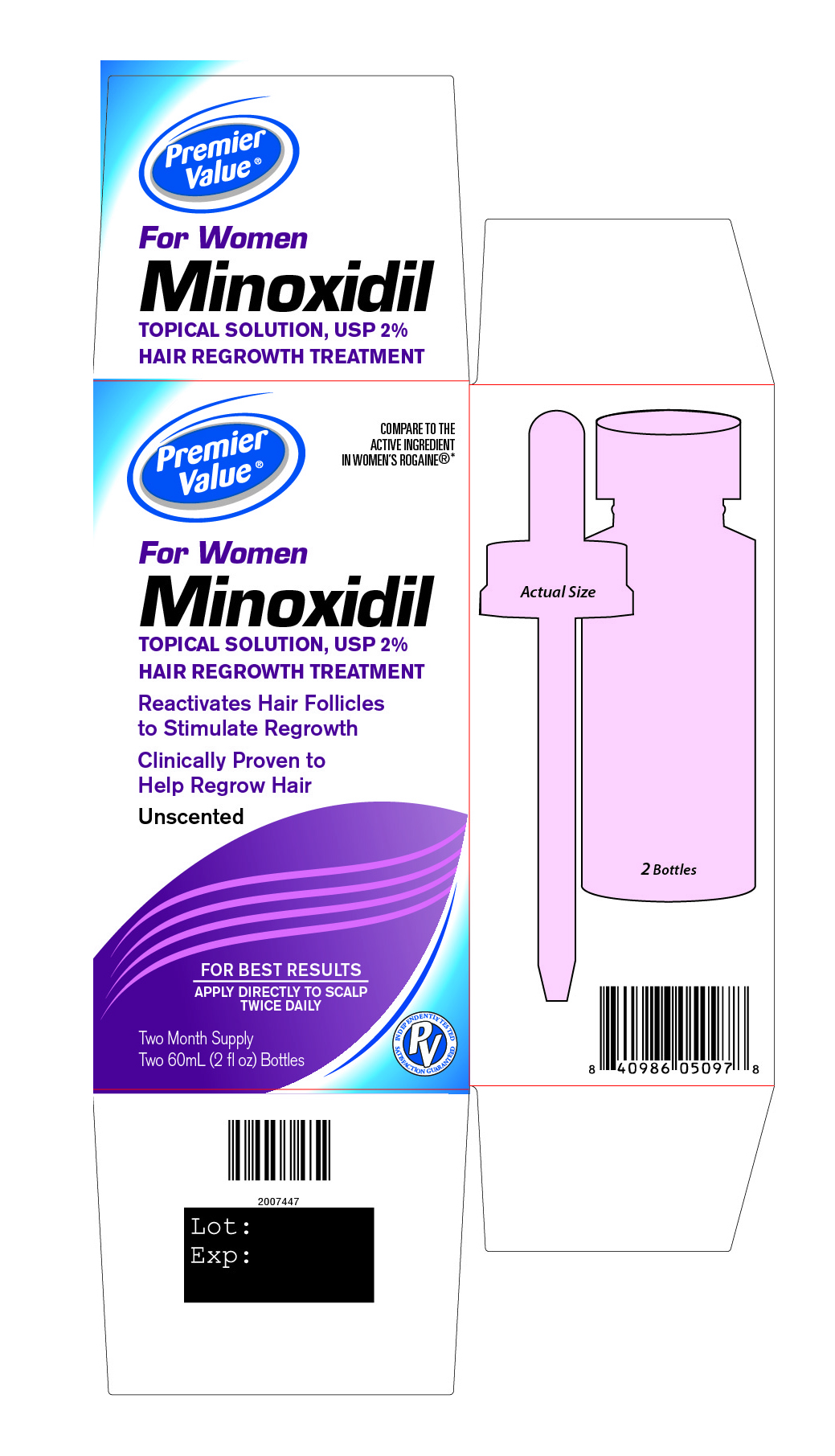
-
INGREDIENTS AND APPEARANCE
MINOXIDIL FOR WOMEN
minoxidil solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-006-64 2 in 1 CARTON 01/01/2000 1 60 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074588 01/01/2000 Labeler - Chain Drug Consortium, LLC (101668460)