TRANSDERM SCOP- scopolamine patch, extended release
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Transderm Scōp® safely and effectively. See full prescribing information for Transderm Scōp®.
Transderm Scōp® (scopolamine) transdermal system patch Initial U.S. Approval: 1979 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONDO NOT cut the patch. Apply ONE patch in the postauricular area to prevent:
DOSAGE FORMS AND STRENGTHSContinuous release, circular, flat, tan colored patch (1.5 mg scopolamine) (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions during motion sickness clinical trials are dry mouth, drowsiness and blurred vision. (6.1) Most common adverse reactions during PONV trials (≥ than 3%) are dry mouth, dizziness, somnolence, urinary retention, agitation, visual impairment, confusion, mydriasis and pharyngitis. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Novartis Consumer Health, Inc. at 1-800-452-0051 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Motion Sickness
Transderm Scōp® is indicated in adults for prevention of nausea and vomiting associated with motion sickness.[see Clinical Studies (14.1)]
1.2 Post Operative Nausea and Vomiting (PONV)
Transderm Scōp® is indicated in adults for prevention of nausea and vomiting associated with recovery from anesthesia and/or opiate analgesia and surgery. [see Clinical Studies (14.2)]
2 DOSAGE AND ADMINISTRATION
Each Transderm Scōp® patch is formulated to deliver in-vivo approximately 1mg of scopolamine over 3 days. Only one patch should be worn at any time. Do not cut the patch.
The patch should be applied only to the skin in the postauricular (hairless area behind one ear) area.
Handling
After the patch is applied on the dry skin behind the ear, the hands should be washed thoroughly with soap and water and dried. Upon removal, the patch should be discarded. To prevent any traces of scopolamine from coming into direct contact with the eyes, after administration of the patch, the hands and the application site should be washed thoroughly with soap and water and dried. [see How Supplied/Storage and Handling (16) and Patient Counseling Information (17)]
2.1 Initiation of Therapy
Motion sickness
- •
- To prevent the nausea and vomiting associated with motion sickness, one Transderm Scōp patch (formulated to deliver approximately 1mg of scopolamine over 3 days) should be applied to the hairless area behind one ear at least 4 hours before the antiemetic effect is required.
Post Operative Nausea and Vomiting
- •
- To prevent post operative nausea and vomiting, one Transderm Scōp patch should be applied the evening before scheduled surgery, except for caesarian section.
For caesarian section surgery, to minimize exposure of the newborn baby to the drug, apply the patch one hour prior to caesarian section
2.2 Continuation of Therapy
Should the patch become displaced, it should be discarded, and a fresh one placed on the hairless area behind the other ear.
Motion Sickness
If therapy is required for longer than 3 days, the first patch should be removed and a fresh one placed on the hairless area behind the other ear.
Post Operative Nausea and Vomiting
For perioperative use, the patch should be kept in place for 24 hours following surgery at which time it should be removed and discarded.
3 DOSAGE FORMS AND STRENGTHS
The Transderm Scōp system is a tan-colored circular flat patch which contains 1.5 mg of scopolamine base and is formulated to deliver in-vivo approximately 1mg of scopolamine over 3 days.
4 CONTRAINDICATIONS
Transderm Scōp is contraindicated in the following populations:
- •
- Patients with angle closure glaucoma. [see Adverse Reactions (6)]
- •
- Persons who are hypersensitive to the drug scopolamine or other belladonna alkaloids or to any ingredient or component in the formulation or delivery system. [see Drug Interactions (7) and Description (11)]
5 WARNINGS AND PRECAUTIONS
5.1 Open Angle Glaucoma
Patients currently being treated for Open Angle Glaucoma
Glaucoma therapy in patients with open angle glaucoma should be monitored and may need to be adjusted during Transderm Scōp use, as the mydriatic effect of scopolamine may cause an increase in intraocular pressure.
Patients should be advised to remove the patch immediately and promptly contact a physician in the event that they experience symptoms of acute angle closure glaucoma (pain and reddening of the eyes, accompanied by dilated pupils).
5.2 Temporary Dilation of the Pupil
Scopolamine can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes.
Patients should be strongly advised to wash their hands thoroughly with soap and water immediately after handling the patch. [see Adverse Reactions (6)] In addition, it is important that used patches be disposed of properly to avoid contact with children or pets. [see How Supplied/ Storage and Handling (16)]
5.3 Preexisting Gastrointestinal or Urinary Bladder Obstructions
Transderm Scōp should be used with caution in patients with pyloric obstruction or urinary bladder neck obstruction. Caution should be exercised when administering an antiemetic or anticholinergic drug, including
Transderm Scōp, to patients suspected of having intestinal obstruction.
Patients should be instructed to remove the patch if they develop any difficulties in urinating. [see Adverse Reactions (6)]
5.4 History of Seizures or Psychosis
Transderm Scōp should be used with caution in patients with a history of seizures or psychosis since scopolamine can potentially aggravate both disorders.
5.5 Idiosyncratic Reactions
Idiosyncratic reactions may occur with ordinary therapeutic doses of scopolamine. The most serious of these that have been reported are: acute toxic psychosis, including confusion, agitation, speech disorder, hallucinations, paranoia, and delusions. [see Adverse Reactions (6)]
5.6 Specific Populations
Pediatric
A safe and effective dose has not been established in the pediatric population [see Use in Specific Populations(8.4)]. Children are particularly susceptible to the side effects of belladonna alkaloids; including mydriasis, hallucinations, amyblyopia, and drug withdrawal syndrome. Neurologic and psychiatric adverse reactions, such as hallucinations, amblyopia and mydriasis have also been reported when one half or one quarter of a patch has been applied.
Elderly
Transderm Scōp should be used with caution in the elderly because of the increased likelihood of CNS effects, such
as hallucinations, confusion, dizziness and drug withdrawal syndrome. Clinical trials of Transderm Scop did not
include sufficient number of subjects aged 65 years and older to determine if they respond differently from younger
subjects. [see Use in Specific Populations (8.5)]
Renal and Hepatic Impaired
Transderm Scōp should be used with caution in individuals with impaired renal or hepatic functions because of the
increased likelihood of CNS effects. Transderm Scop has not been studied in these populations. [see Use in Specific Populations (8.6)]
5.7 Safety Hazards
Drowsiness
Since drowsiness, disorientation, and confusion may occur with the use of scopolamine, patients should be warned of the possibility and cautioned against engaging in activities that require mental alertness, such as driving a motor vehicle or operating dangerous machinery.
Disorienting Effects
Patients who expect to participate in underwater sports should be cautioned regarding the potentially disorienting effects of scopolamine. [see Patient Counseling Information (17)]
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical
trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates
observed in clinical practice.
Motion Sickness
In motion sickness clinical studies of Transderm Scōp, the most frequent adverse reaction was dry mouth. This occurred in about two thirds of patients on drug. A less frequent adverse drug reaction was drowsiness, which occurred in less than one sixth of patients on drug. Transient impairment of eye accommodation, including blurred vision and dilation of the pupils, was also observed.
Post-Operative Nausea and Vomiting
In a total of five clinical studies in which Transderm Scōp was administered perioperatively to a total of 461 patients where safety was assessed, dry mouth was the most frequently reported adverse drug reaction, which occurred in approximately 29% of patients on drug. Dizziness was reported by approximately 12% of patients on drug. Other adverse drug reactions reported from these studies, with a frequency of ≥3% of patients treated with Transderm Scop and with a frequency higher than placebo were, in descending order: somnolence, urinary retention, agitation/restlessness, visual impairment, confusion, mydriasis and pharyngitis (see Table 6.1).
|
Transderm Scōp (N=461) |
Placebo (N=457) |
|||
|
n |
% |
n |
% |
|
|
Adverse Drug Reactions |
303 |
65.7 |
259 |
56.7 |
|
Dry mouth |
133 |
28.9 |
72 |
15.8 |
|
Dizziness |
57 |
12.4 |
33 |
7.2 |
|
Somnolence |
36 |
7.8 |
16 |
3.5 |
|
Urinary Retention |
33 |
7.2 |
30 |
6.6 |
|
Agitation |
28 |
6.1 |
20 |
4.4 |
|
Visual Impairment |
23 |
5.0 |
12 |
2.6 |
|
Confusion |
18 |
3.9 |
14 |
3.1 |
|
Mydriasis |
16 |
3.5 |
2 |
0.4 |
|
Pharyngitis |
15 |
3.3 |
10 |
2.2 |
6.2 Postmarketing Experience
The following adverse drug reactions, further to those reported from clinical trials, have been identified during postapproval use of Transderm Scop. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to confirm a definite causal relationship.
In worldwide marketing with Transderm Scōp, the following adverse drug reactions were reported by body system.
Psychiatric disorders: acute psychosis including: hallucinations disorientation, and paranoia.
Nervous system disorders: headache, amnesia, coordination abnormalities, speech disorder, disturbance in attention,
restlessness.
General disorders and administration site conditions: application site burning.
Eye disorders: dry eyes, eye pruritis, angle closure glaucoma, amblyopia, eyelid irritation.
Skin and subcutaneous tissue disorders: rash generalized, skin irritation, erythema.
Renal and urinary disorders: dysuria.
Ear and Labyrinth Disorders: vertigo.
6.3 Drug Withdrawal/Post-Removal Symptoms
Symptoms such as dizziness, nausea, vomiting, abdominal cramps, sweating, headache mental confusion, muscle weakness, bradycardia and hypotension may occur following abrupt discontinuation of anticholinergic drugs such as Transderm Scōp. Similar symptoms, including disturbances of equilibrium, have been reported in some patients following discontinuation of use of the Transderm Scōp system. These symptoms usually do not appear until 24 hours or more after the patch has been removed. These symptoms can be severe and may require medical intervention. Some symptoms may be related to adaptation from a motion environment to a motion-free environment.
These symptoms can be severe and may require medical intervention.
7 DRUG INTERACTIONS
The absorption of oral medications may be decreased during the concurrent use of scopolamine because of
decreased gastric motility and delayed gastric emptying. [see Warnings and Precautions (5.3)]
Scopolamine should be used with caution in patients taking other drugs that are capable of causing CNS effects such
as sedatives, tranquilizers, or alcohol. Special attention should be paid to potential interactions with drugs having
anticholinergic properties; e.g., other belladonna alkaloids, antihistamines (including meclizine), tricyclic
antidepressants, and muscle relaxants.
In vitro studies indicated that the potential for scopolamine to alter the pharmacokinetics of other concomitant
medications through inhibition of CYP 1A2, 2C8, 2C9, 2C19, 2D6 and 3A4 or induction of CYP 1A2 and 3A4 is
low; however, in vivo studies have not been conducted. [see Clinical Pharmacology (12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Based on data from one prospective study of Transderm Scōp in cesarean delivery, the rate of newborn adverse
events in both the Transderm Scōp and placebo groups were the same. The rates were 10.5% (12 events in 114
newborns) in both treatment groups. None of these events were considered life threatening or drug related.
Jaundice was the only adverse event occurring more frequently with Transderm Scōp than placebo: 9 events (7.9%)
versus 2 events (1.8%) (p=0.031). Jaundice, a common occurrence in newborns, resolved with ultraviolet light and
did not prolong the hospital stay.
There are no adequate and well-controlled studies of Transderm Scōp use during pregnancy. In animal reproduction
studies, when pregnant rats and rabbits received scopolamine hydrobromide by daily intravenous injection, no
adverse effects were observed in rats. An embryotoxic effect was observed in rabbits at doses producing plasma
levels approximately 100 times the levels achieved in humans using a transdermal system. Transderm Scōp should
be used during pregnancy only if the potential benefit justifies the potential risk to the fetus and the mother.
8.2 Labor and Delivery
During a clinical study among women undergoing cesarean section treated with Transderm Scōp in conjunction with
epidural anesthesia and opiate analgesia, no evidence of CNS depression was found in newborns. [see Clinical Studies (14.2)]Scopolamine administered parenterally to rats and rabbits at doses higher than the dose delivered by
Transderm Scōp did not affect uterine contractions or increase the duration of labor. Scopolamine does cross the
placenta.
8.3 Nursing Mothers
Scopolamine is excreted in human milk. Caution should be exercised when Transderm Scōp is administered to a
nursing woman.
8.4 Pediatric Use
A safe and effective dose has not been established in the pediatric population. [see Warnings and Precautions (5.6)]
8.5 Geriatric Use
Transderm Scōp should be used with caution in the elderly because of the increased likelihood of CNS effects, such
as hallucinations, confusion and dizziness. Clinical trials of Transderm Scop did not include sufficient number of
subjects aged 65 years and older to determine if they respond differently from younger subjects. [see Warnings and Precautions (5.6)]
8.6 Renal or Hepatic Impairment
Transderm Scōp should be used with caution in individuals with impaired renal or hepatic functions because of the increased likelihood of CNS effects. [see Warnings and Precautions (5.6)]
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Scopolamine is an antagonist at muscarinic receptors in the cholinergic system. Drugs in this class are not known to
have significant abuse potential in humans.
9.3 Dependence
Abrupt termination of Transderm Scōp may result in withdrawal symptoms such as dizziness, nausea, vomiting,
abdominal cramps, sweating, headache, mental confusion, muscle weakness, bradycardia and hypotension. [see Adverse Reaction (6.3) and Overdosage (10)] These withdrawal symptoms indicate that anticholinergic drugs, like
scopolamine may produce physical dependence. These symptoms can be severe and may require medical
intervention.
10 OVERDOSAGE
Because strategies for the management of drug overdose continually evolve, it is strongly recommended that a
poison control center be contacted to obtain up-to-date information regarding the management of Transderm Scōp®
patch overdose. The prescriber should be mindful that antidotes used routinely in the past may no longer be
considered optimal treatment. For example, physostigmine, used more or less routinely in the past, is seldom
recommended for the routine management of anticholinergic syndromes.
Until up-to-date authoritative advice is obtained, routine supportive measures should be directed to maintaining
adequate respiratory and cardiac function.
The signs and symptoms of anticholinergic toxicity include: lethargy, somnolence, coma, confusion, agitation,
hallucinations, convulsion, visual disturbance, dry flushed skin, dry mouth, decreased bowel sounds, urinary
retention, tachycardia, hypertension, and supraventricular arrhythmias. These symptoms can be severe and may
require medical intervention.
In cases of toxicity remove the patch. Serious symptomatic cases of overdosage involving multiple patch
applications and/or ingestion may be managed by initially ensuring the patient has an adequate airway, and
supporting respiration and circulation. This should be rapidly followed by removal of all patches from the skin and
the mouth. If there is evidence of patch ingestion, gastric lavage, endoscopic removal of swallowed patches, or
administration of activated charcoal should be considered, as indicated by the clinical situation. In any case where
there is serious overdosage or signs of evolving acute toxicity, continuous monitoring of vital signs and ECG,
establishment of intravenous access, and administration of oxygen are all recommended.
The symptoms of overdose/toxicity due to scopolamine should be carefully distinguished from the occasionally
observed syndrome of withdrawal. [see Adverse Reactions (6.3)] Although mental confusion and dizziness may be
observed with both acute toxicity and withdrawal, other characteristic findings differ: tachyarrhythmias, dry skin,
and decreased bowel sounds suggest anticholinergic toxicity, while bradycardia, headache, nausea and abdominal
cramps, and sweating suggest post-removal withdrawal. Obtaining a careful history is crucial to making the correct
diagnosis.
11 DESCRIPTION
The Transderm Scōp® (scopolamine) transdermal system is a circular flat patch designed for continuous release of
scopolamine following application to an area of intact skin on the head, behind the ear. Each system contains 1.5 mg
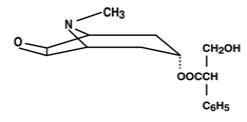
of scopolamine base. Scopolamine is α -(hydroxymethyl) benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo
[3.3.1.0 2,4] non-7-yl ester. The empirical formula is C17H21NO4 and its structural formula is:
Scopolamine is a viscous liquid that has a molecular weight of 303.35 and a pKa of 7.55-7.81. The Transderm Scōp
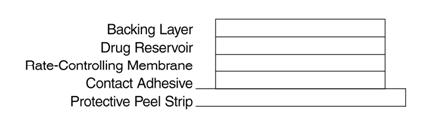
system is a film 0.2 mm thick and 2.5 cm2, with four layers. Proceeding from the visible surface towards the surface
attached to the skin, these layers are: (1) a backing layer of tan-colored, aluminized, polyester film; (2) a drug
reservoir of scopolamine, light mineral oil, and polyisobutylene; (3) a microporous polypropylene membrane that
controls the rate of delivery of scopolamine from the system to the skin surface; and (4) an adhesive formulation of
mineral oil, polyisobutylene, and scopolamine. A protective peel strip of siliconized polyester, which covers the
adhesive layer, is removed before the system is used. The inactive components, light mineral oil (12.4 mg) and
polyisobutylene (11.4 mg), are not released from the system.
Cross section of the system:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Scopolamine, a belladonna alkaloid, is an anticholinergic agent. Scopolamine acts: i) as a competitive inhibitor at
postganglionic muscarinic receptor sites of the parasympathetic nervous system, and ii) on smooth muscles that
respond to acetylcholine but lack cholinergic innervation. It has been suggested that scopolamine acts in the central
nervous system (CNS) by blocking cholinergic transmission from the vestibular nuclei to higher centers in the CNS
and from the reticular formation to the vomiting center. Scopolamine can inhibit the secretion of saliva and sweat,
decrease gastrointestinal secretions and motility, cause drowsiness, dilate the pupils, increase heart rate, and depress
motor function.
12.3 Pharmacokinetics
The pharmacokinetics of scopolamine delivered via the system are due to the characteristics of both the drug and
dosage form. The system is formulated to deliver in-vivo approximately 1 mg of scopolamine at an approximately
constant rate to the systemic circulation over 3 days. Upon application to the postauricular skin, an initial priming
dose of scopolamine is released from the adhesive layer to saturate skin-binding sites. The subsequent delivery of
scopolamine to the blood is determined by the rate controlling membrane and is designed to produce stable plasma
levels in a therapeutic range. Following removal of the used system, there is some degree of continued systemic
absorption of scopolamine bound in the skin layers.
Absorption
Scopolamine is well absorbed percutaneously. Following application to the skin behind the ear, circulating plasma
levels are detected within 4 hours with peak levels being obtained, on average, within 24 hours. The average plasma
concentration produced is 87 pg/mL (0.28 nM) for free scopolamine and 354 pg/mL for total scopolamine (free +
conjugates).
Distribution
The distribution of scopolamine is not well characterized. It crosses the placenta and the blood brain barrier and may be reversibly bound to plasma proteins.
Metabolism and Excretion
The exact elimination pattern of scopolamine has not been determined. Following patch removal, plasma levels of scopolamine decline in a log linear fashion with an observed half-life of 9.5 hours. Less than 10% of the total dose is excreted in the urine as the parent drug and metabolites over 108 hours. Scopolamine is extensively metabolized and conjugated with less than 5% of the total dose appearing unchanged in the urine. The enzymes responsible for metabolizing scopolamine are unknown.
Drug Interaction
An in vitro study using human hepatocytes examined the induction of CYP1A2 and CYP3A4 by scopolamine.
Scopolamine did not induce CYP1A2 and CYP3A4 isoenzymes at the concentrations up to 10 nM.
In an in vitro study using human liver microsomes which evaluated the inhibition of CYP1A2, 2C8, 2C9, 2C19, 2D6
and 3A4, scopolamine did not inhibit these cytochrome P450 isoenzymes at the concentrations up to 1 mcM.
13 NONCLINICAL PHARMACOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been conducted to evaluate the carcinogenic potential of scopolamine. The
mutagenic potential of scopolamine has not been evaluated.
Fertility studies were performed in female rats and revealed no evidence of impaired fertility or harm to the fetus
due to scopolamine hydrobromide administered by daily subcutaneous injection. Maternal body weights were
reduced in the highest-dose group (plasma level approximately 500 times the level achieved in humans using a
transdermal system). However, fertility studies in male animals were not performed.
14 CLINICAL STUDIES
14.1 Motion Sickness
In 195 adult subjects of different racial origins who participated in clinical efficacy studies at sea or in a controlled motion environment, there was a 75% reduction in the incidence of motion-induced nausea and vomiting.
14.2 Post-Operative Nausea and Vomiting
In two pivotal clinical efficacy studies in 391 adult female patients undergoing cesarean section or gynecological
surgery with anesthesia and opiate analgesia, 66% of those treated with Transderm Scōp® (compared to only 46% of
those receiving placebo) reported no retching/vomiting within the 24-hour period following administration of
anesthesia/opiate analgesia. When the need for additional antiemetic medication was assessed during the same
period, there was no need for medication in 76% of patients treated with Transderm Scōp as compared to 59% of
placebo-treated patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
The Transderm Scōp® system is a tan-colored circular patch, 2.5 cm2, on a clear, oversized, hexagonal peel strip,
which is removed prior to use.
Each Transderm Scōp system contains 1.5 mg of scopolamine and is formulated to deliver in-vivo approximately
1 mg of scopolamine over 3 days. Transderm Scōp is available in packages of four patches. Each patch is foil
wrapped. Patient instructions are included. [see Patient Counseling Information (17)]
- •
- 1 Package (4 patches) NDC 0067-4345-04
Storage
The system should be stored at controlled room temperature between 20°C-25°C (68°F-77°F).
Handling
Since scopolamine can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes,
patients should be strongly advised to wash their hands thoroughly with soap and water immediately after handling
the patch. In addition, it is important that used patches be disposed of properly to avoid contact with children or pets.
[see Dosage and Administration (2), Warnings and Precautions (5.2), and Patient Counseling Information (17))]
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Please read this instruction sheet carefully before opening the system package.
Transderm Scōp® Transdermal System
Generic Name: scopolamine, pronounced skoe-POL-a-meen
- •
- Elderly patients should be informed that Transderm Scōp may cause a greater likelihood of CNS effects, such as hallucinations, confusion, dizziness and drug withdrawal syndrome and to seek immediate medical care if they become confused, disoriented or dizzy while wearing the patch or after removing.
- •
- Patients should be informed that since Transderm Scōp may cause drowsiness, disorientation and confusion they should avoid engaging in activities that require mental alertness such as driving a motor vehicle or operating dangerous machinery.
- •
- Patients who expect to participate in underwater sports should be cautioned regarding the potentially disorienting effects of Transderm Scop.
- •
- Because of the possibility of drowsiness, disorientation and confusion, patients should be informed that they should avoid drinking alcohol. In addition, patients should be informed that the following medications should be used with caution when taking Transderm Scop:
- •
- sedatives or tranquilizers
- •
- drugs with anticholinergic properties (e.g., other belladonna alkaloids),
- •
- antihistamines (including meclizine)
- •
- tricyclic antidepressants
- •
- muscle relaxants
- •
- Patients with the following conditions should be informed about the chance of developing serious reactions with Transderm Scōp:
- •
- patients with open angle glaucoma (may cause an increase in intraocular pressure)
- •
- patients with impaired kidney or liver function (increased likelihood of CNS effects)
- •
- patients with a history of seizures or psychosis (can potentially worsen both disorders)
- •
- patients with obstruction at the level of the pylorus, which is the outlet of the stomach, or urinary bladder neck obstruction (may cause difficulties in urinating)
- •
- patients suspected of having intestinal obstruction
- •
- pregnant or nursing mothers
- •
- Patients should be informed that if they remove the Transderm Scōp patch suddenly before treatment is complete, the following withdrawal symptoms may occur: dizziness, nausea, vomiting, abdominal cramps, sweating, headache, mental confusion, muscle weakness, slow heart rate and low blood pressure. Patients should be instructed to seek immediate medical care if they develop any of these symptoms after removing Transderm Scōp.
- •
- Patients should be informed that Transderm Scop can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes. Patients should be informed to wash their hands thoroughly with soap and water immediately after handling the patch. In addition, patients should be informed that used patches must be disposed of properly to avoid contact with children or pets.
- •
- Patients should be informed that skin burns have been reported at the patch site in several patients wearing an aluminized transdermal system during a Magnetic Resonance Imaging scan (MRI). Because Transderm Scōp contains aluminum, patients should be advised to remove the system before undergoing an MRI.
- •
- Patients should be advised to use only one patch at a time.
- •
- Patients should be advised not to cut the patch.
PATIENT INFORMATION
Transderm Scōp®, pronounced tran(t)s-derm skōp
(scopolamine)
transdermal system patch
Read this Patient Information before you start using Transderm Scōp® and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is Transderm Scōp®?
The Transderm Scōp® patch is a prescription medicine used for adults to:
- •
- help prevent nausea and vomiting from motion sickness
- •
- help prevent nausea and vomiting from anesthesia or taking opioid pain medicines after surgery
It is not known if Transderm Scōp® is safe or effective in children.
Who should not use Transderm Scōp®?
Do not use Transderm Scōp® if you:
- •
- have an eye problem called angle closure glaucoma
- •
- if you are allergic to any of the ingredients in Transderm Scōp® or other medicines called belladonna alkaloids. See the end of this leaflet for a list of the ingredients in Transderm Scōp®. Ask your doctor if you are not sure.
What should I tell my doctor before using Transderm Scōp®?
Before you use Transderm Scōp®, tell your doctor if you:
- •
- are scheduled to have a gastric secretion test
- •
- have glaucoma (increased pressure in the eye)
- •
- have liver or kidney problems
- •
- have problems with your stomach or intestines
- •
- have trouble urinating
- •
- have a history of seizures or psychosis
- •
- have any other medical conditions
- •
- are pregnant or plan to become pregnant. It is not known if Transderm Scōp® can harm your unborn baby.
- •
- are breast-feeding or plan to breast-feed. Transderm Scōp® can pass into your breast milk and may harm your baby. Talk to your doctor about the best way to feed your baby if you use Transderm Scōp®.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Transderm Scōp® may affect the way other medicines work, and other medicines may affect how Transderm Scōp® works. Medicines that you take by mouth may not be absorbed well while you use Transderm Scōp®.
Especially tell your doctor if you take:
- •
- a sedative or tranquilizer (medicines that make you sleepy)
- •
- an antidepressant medicine
- •
- an anticholinergic medicine, such as an allergy or cold medicine, a medicine to treat bladder or bowel spasms, certain asthma medicines, or other medicines for motion sickness.
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I use Transderm Scōp®?
- Use Transderm Scōp® exactly as your doctor tells you to use it.
- Transderm Scōp® is a tan-colored circle shaped patch.
- Wear only one patch at any time.
- Do not cut the patch.
To help prevent nausea and vomiting from motion sickness:
- •
- Apply one Transderm Scōp® patch to your skin on a hairless area behind one ear at least 4 hours before the activity to prevent nausea and vomiting.
- •
- If the treatment is needed for longer than 3 days, remove the patch from the hairless area behind your ear. Get a new Transderm Scōp® patch and place it on the hairless area behind your other ear.
To help prevent nausea and vomiting after surgery:
- •
- Follow your doctor’s instructions about when to apply Transderm Scōp® before your scheduled surgery.
- •
- The Transderm Scōp® patch should be left in place for 24 hours after surgery. After 24 hours the patch should be removed and thrown away.
Apply Transderm Scōp® as follows:
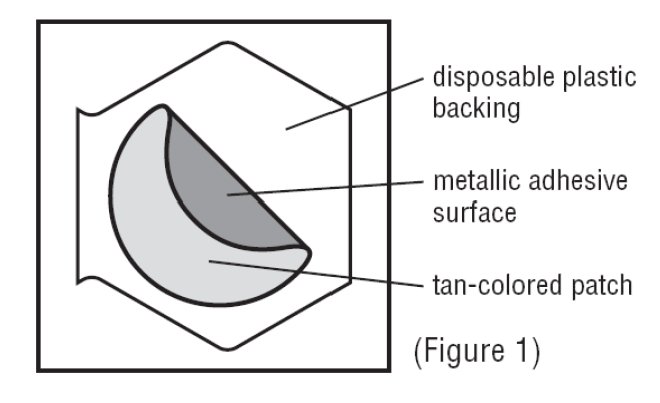
Inside the Transderm Scōp® package, you will find one Transderm Scōp® patch. A tan colored patch with a metallic (silver) sticky surface is adhered to a clear disposable backing (See Figure 1).
1. Select a hairless area of skin behind one of your ears. Avoid areas on your skin that may have cuts, pain or tenderness. Wipe the area of your skin with a clean, dry tissue.
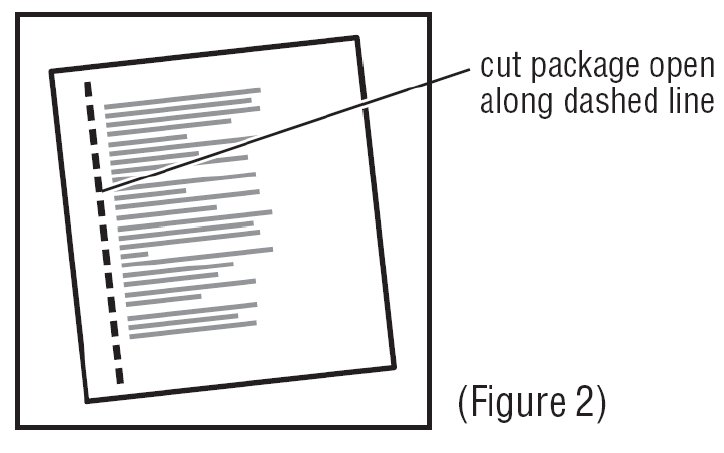
2. Cut along dotted line on the Transderm Scōp® package to open (See Figure 2).
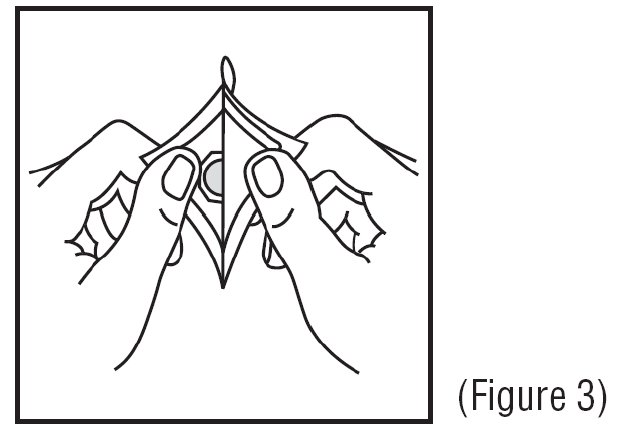
3. Remove the clear plastic backing from the tan-colored round patch (See Figure 3).
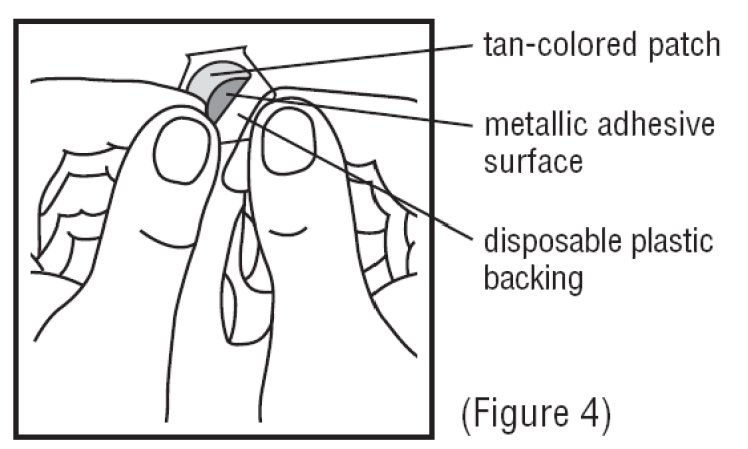
4. Avoid touching the metallic adhesive (sticky) surface on the patch with your hands (See Figure 4).
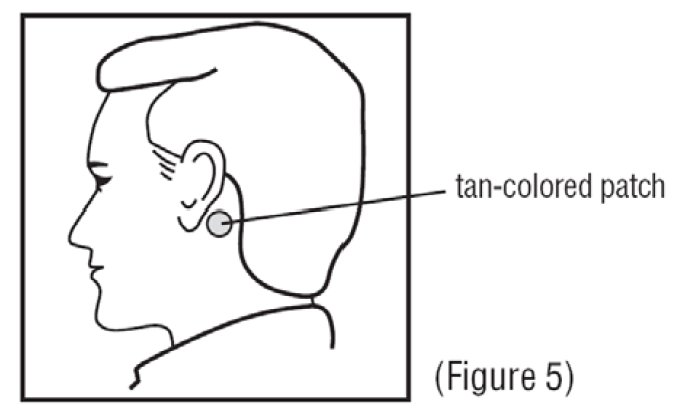
5. Apply the metallic adhesive surface of the patch firmly to the dry area of skin behind your ear. The tan-colored side of the patch should be facing up and showing (See Figure 5). Wash your hands with soap and water right away after applying the patch, so that any medicine from the patch that gets on your hands
will not get into your eyes.
After removing the patch, be sure to wash your hands and the area behind your ear thoroughly with soap and water. Note that the used patch will still contain some of the active ingredient after use. To avoid accidental contact or ingestion by children or pets, fold the used patch in half with the sticky side together. Dispose in the trash out of the reach of children and pets.
If you use too much Transderm Scōp®, call your doctor or local poison control center, or go to the nearest hospital emergency room right away.
What should I avoid while using Transderm Scōp®?
- •
- You should not drink alcohol while using Transderm Scōp®. It can increase your chances of having serious side effects.
- •
- You should not drive, operate heavy machinery, or do other dangerous activities until you know how Transderm Scōp® affects you.
- •
- You should not use Transderm Scōp® during a Magnetic Resonance Imaging scan (MRI). Remove Transderm Scōp® patch before undergoing an MRI; it can cause your skin to burn.
- •
- You should be careful if you use Transderm Scōp® while you participate in watersports because you may feel lost or confused (disoriented).
- •
- Limit contact with water while swimming and bathing because the Transderm Scōp® patch may fall off. If the patch falls off, throw it away and apply a new one on the hairless area behind your other ear.
What are the possible side effects of Transderm Scōp®?
Transderm Scōp® may cause serious side effects, including:
- •
- angle closure glaucoma. If you have open angle glaucoma and use Transderm Scōp®, remove the patch and call a doctor right away if you get pain and reddening of your eyes with an increase in the size of your pupil (the small dark circle in the eye).
- •
- temporary increase in the size of your pupil and blurry vision, especially if Transderm Scōp® comes in contact with your eyes
- •
- difficulties in urinating
- •
- difficulties in food passing from the stomach to the small intestines, which may cause abdominal pain, nausea or vomiting.
- •
- worsening of seizures. Tell your doctor about any worsening of seizures while using Transderm Scōp®.
- •
-
an unusual reaction called acute psychosis: Tell your doctor if you have any of these symptoms:
- •
- confusion
- •
- agitation
- •
- rambling speech
- •
- hallucinations (seeing or hearing things that are not there)
- •
- paranoid behaviors and delusions (false belief in something)
- •
- skin burns at the site of the patch. This can happen during a medical test called a Magnetic Resonance Imaging scan (MRI). Transderm Scōp® contains aluminum and should be removed from your skin before you have an MRI.
The most common side effects of using Transderm Scōp® include:
- •
- dry mouth
- •
- drowsiness
- •
- disorientation (confusion)
- •
- blurred vision
- •
- pharyngitis
- •
- memory trouble
- •
- dizziness
- •
- restlessness
- •
- agitation
- •
- problems urinating
- •
- skin rashes or redness, application site burning
- •
- dry itchy, or reddened whites of the eyes, and eye pain
Symptoms when removing Transderm Scōp®. Some people may have certain symptoms 24 hours or more after removing Transderm Scōp®. These symptoms may include:
- •
- dizziness
- •
- nausea
- •
- vomiting
- •
- headache
- •
- problems with balance and walking
- •
- decrease in blood pressure
- •
- muscle weakness
- •
- decrease in heart rate
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Transderm Scōp®. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may reports side effects to FDA at 1-800-FDA-1088.
How should I store Transderm Scōp®?
- •
- Store Transderm Scōp® at room temperature between 68°F and 77°F (20°C and 25°C) until you are ready to use it.
- •
- Keep Transderm Scōp® and all medicines out of reach of children.
General Information about Transderm Scōp®
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use Transderm Scōp® for a condition for which it was not prescribed. Do not give Transderm Scōp® to other people, even if they have the same symptoms you have. It may harm them.
This patient information leaflet summarizes the most important information about Transderm Scōp®. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Transderm Scōp® that is written for the health professionals.
For more information, go to www. transdermscop.com or call 1-800-452-0051.
What are the ingredients in the Transderm Scōp® patch?
Active ingredient: Scopolamine
Inactive ingredients: light mineral oil and polyisobutylene and aluminized polyester film
Manufactured by: ALZA Corporation
Vacaville, CA 95688
Distributed by:
Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622
©2013
| TRANSDERM SCOP
scopolamine patch, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |