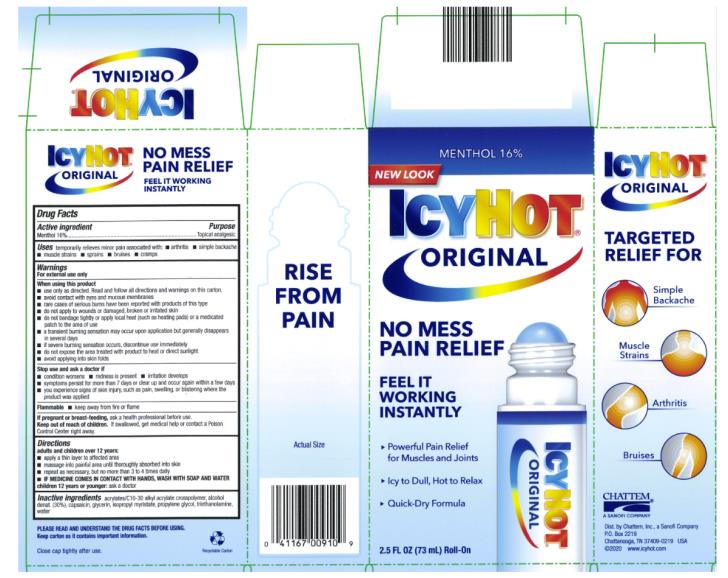
Label: ICY HOT MEDICATED NO MESS APPLICATOR- menthol liquid
- NDC Code(s): 41167-0091-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- avoid contact with eyes and mucous membranes
- rare cases of serious burns have been reported with products of this type
- do not apply to wounds or damaged, broken or irritated skin
- do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
- a transient burning sensation may occur upon application but generally disappears in several days
- if severe burning sensation occurs, discontinue use immediately
- do not expose the area treated with product to heat or direct sunlight
- avoid applying into skin folds
Stop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Flammable ■ Keep away from fire or flame
- use only as directed. Read and follow all directions and warnings on this carton.
- Directions
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, capsaicin, glycerin, isopropyl myristate, propylene glycol, alcohol denat. (30%), triethanolamine, water
Close cap tightly after use. Keep carton as it contains important information.Distributed by Chattem, Inc.
P.O. Box 2219
Chattanooga, TN 37409-0219
U.S.A ©2010 www.icyhot.com Recyclable Carton - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ICY HOT MEDICATED NO MESS APPLICATOR
menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0091 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.16 g in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) CAPSAICIN (UNII: S07O44R1ZM) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0091-0 1 in 1 CARTON 12/01/2009 1 73 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/01/2009 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 830410721 label(41167-0091) , manufacture(41167-0091) , pack(41167-0091) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 003336013 analysis(41167-0091) , label(41167-0091) , manufacture(41167-0091) , pack(41167-0091)