Label: LEGACY- gentamicin solution
- NDC Code(s): 57561-336-04, 57561-336-05
- Packager: Agri Laboratories, Ltd.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated June 16, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
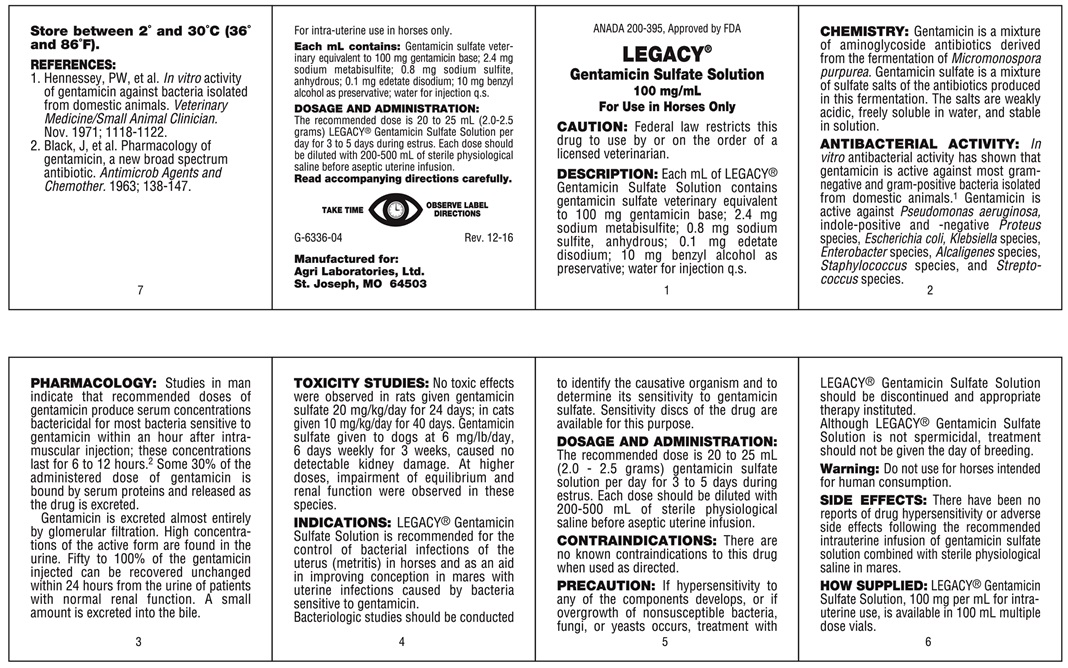
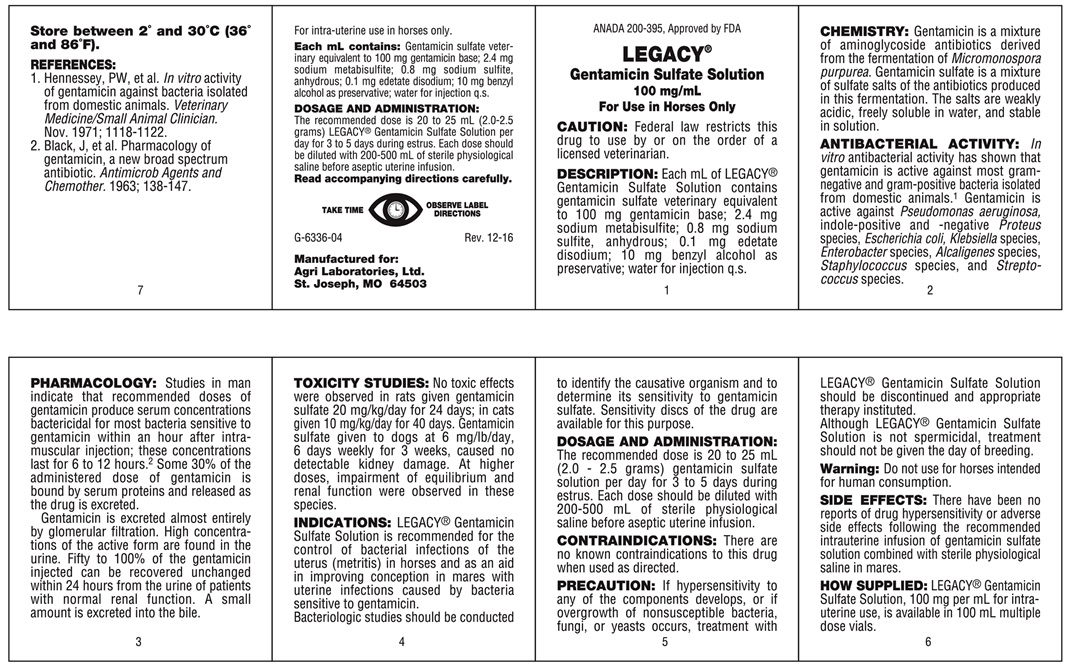
DESCRIPTION
Gentamicin Sulfate Solution
100 mg/mL Gentamicin Sterile Multiple Dose Vial
For Use in Horses Only
For intra-uterine use in horses only.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION: Each mL of LEGACY Gentamicin Sulfate Solution contains gentamicin sulfate veterinary equivalent to 100 mg gentamicin base; 2.4 mg sodium metabisulfite; 0.8 mg sodium sulfite, anhydrous; 0.1 mg edetate disodium; 10 mg benzyl alcohol as preservative; water for injection q.s.
-
CLINICAL STUDIES
CHEMISTRY
Gentamicin is a mixture of aminoglycside antibiotics derived from the fermentation of Micomonospora pupurea. Gentamicin sulfate is a mixture of sulfate salts of the antibiotics produced in this fermentation. The salts are weakly acidic, freely soluble in water, and stable in solution.
ANTIBACTERIAL ACTIVITY
In vitro antibacterial activity has shown that gentamicin is active against most gram-negative and gram-positive bacteria isolated from domestic animals.1 Gentamicin is active against Pseudomonas aeruginosa, indole-positive and -negative Proteus species, Escherichiacoli, Klebsiella species, Enterobactar species, Alcaligenes species, Staphylococcus species, and Streptococcus species.
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PHARMACOLOGY
Studies in man indicate that recommended doses of gentamicin produce serum concentrations bactericidal for most bacteria sensitive to gentamicin within an hour after intramuscular injection; these concentrations last for 6 to 12 hours.2 Some 30% of the administered dose of gentamicin is bound by serum proteins and released as the drug is excreted.
Gentamicin is excreted almost entirely by glomerular filtration. High concentrations of the active form are found in the urine. Fifty to 100% of the gentamicin injected can be recovered unchanged within 24 hours from the urine of patients with normal renal function. A small amount is excreted into the bile.
TOXICITY STUDIES
No toxic effects were observed in rats given gentamicin sulfate 20 mg/kg/day for 24 days; in cats given 10 mg/kg/day for 40 days. Gentamicin sulfate given to dogs at 6 mg/lb/day, 6 days weekly for 3 weeks, caused no detectable kidney damage. At higher doses, impairment of equilibrium and renal function were observed in these species.
-
INDICATIONS
LEGACY Gentamicin Sulfate Solution is recommended for the control of bacterial infections of the uterus (metritis) in horses and as an aid in improving conception in mares with uterine infections caused by bacteria sensitive to gentamicin.
Bacteriologic studies should be conducted to identify the causative organism and to determine its sensitivity to gentamicin sulfate. Sensitivity discs of the drug are available for this purpose.
- DOSAGE AND ADMINISTRATION
- CONTRAINDICATIONS
-
PRECAUTION
If hypersensitivity to any of the components develops, or if overgrowth of nonsusceptible bacteria, fungi, or yeasts occurs, treatment with LEGACY Gentamicin Sulfate Solution should be discontinued and appropriate therapy instituted.
Although LEGACY Gentamicin Sulfate Solution is not spermicidal treatment should not be given the day of breeding
Warning: Do not use for horses intended for human consumption.
- SIDE EFFECTS
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- Each mL contains
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEGACY
gentamicin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:57561-336 Route of Administration INTRAUTERINE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GENTAMICIN SULFATE (UNII: 8X7386QRLV) (GENTAMICIN - UNII:T6Z9V48IKG) GENTAMICIN 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57561-336-04 100 mL in 1 VIAL 2 NDC:57561-336-05 250 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200395 10/07/2008 Labeler - Agri Laboratories, Ltd. (155594450)