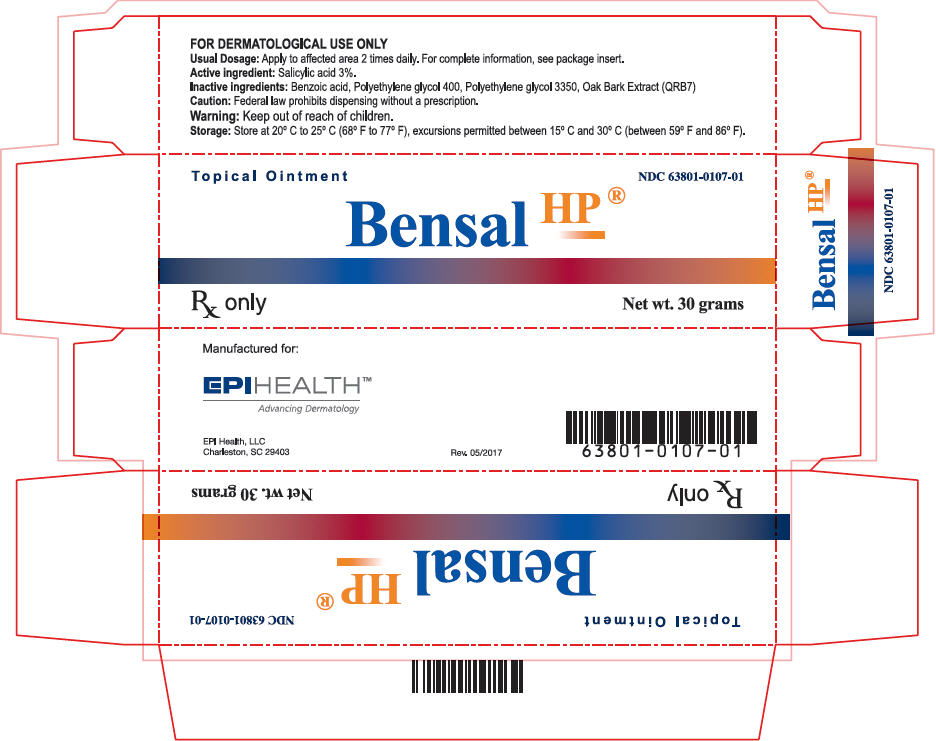
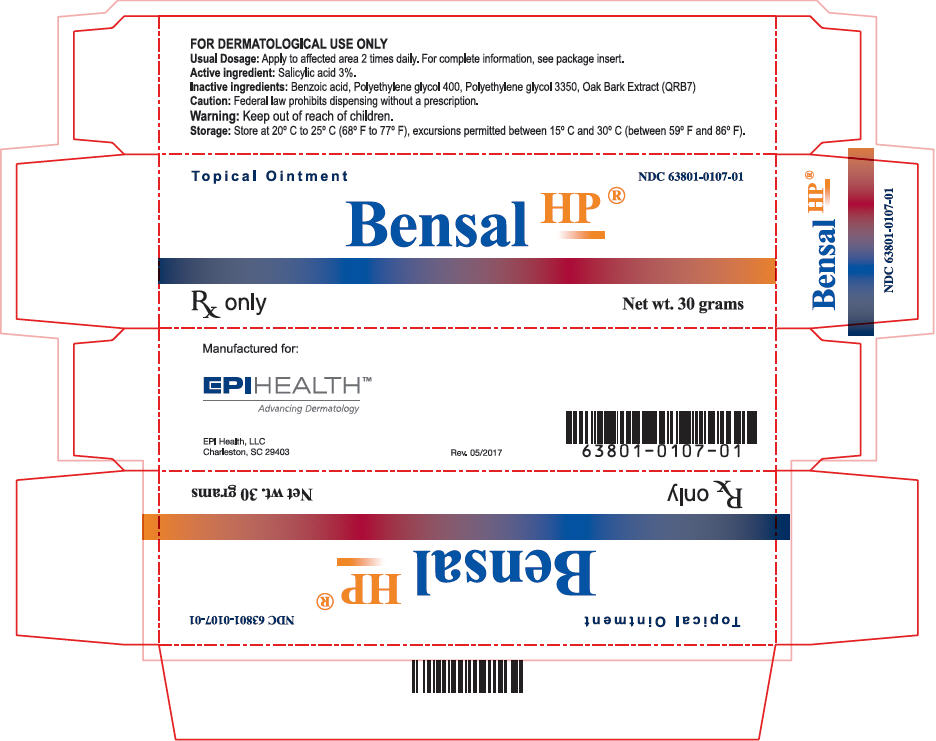
Label: BENSAL HP- salicylic acid ointment
- NDC Code(s): 63801-107-01, 63801-107-15
- Packager: SMG Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
The mechanism of action of Bensal HP® is not known. While the following animal data are available, their clinical significance is unknown. It has been demonstrated that Bensal HP® significantly reduces methicillin-resistant Staphylococcus aureus (MRSA) protected by biofilms in wounds using porcine models. In addition, Bensal HP® stimulates re-epithelialization of second-degree burns in porcine models.
-
CLINICAL STUDIES
A randomized, double-blind, placebo-controlled study evaluated the rate of wound re-epithelialization. Four partial-thickness wounds (2×2 cm & 0.2 mm deep) were created under local anesthesia on the thighs of 13 normal, healthy, male volunteers with an electrokeratome. Bensal HP® substantially increased the rate of re-epithelialization by 63% over the vehicle alone (p<0.01) and 77% over untreated control (p<0.005).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- DRUG INTERACTIONS
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Patients should be advised to follow these step-by-step instructions for application of Bensal HP® Ointment:
Hands should be washed thoroughly.
When using tubes, the tip of the tube should not come into contact with the area to be treated; the tube should be recapped tightly after each application.
If applying with a cotton-tipped applicator, which is recommended, use once and discard.
Bensal HP® Ointment should be applied twice a day for best results.
Gently rinse the area to be treated with saline or water and then pat dry. Bensal HP® Ointment can be applied directly to the wound or placed on dry gauze and then placed on the wound. Wet-Packs or Wet-To-Dry Dressings are not recommended since they will dilute the ointment and decrease its effectiveness. Bensal HP® is designed to provide moisture to the wound.
Spread a generous quantity of Bensal HP® Ointment evenly over the desired area to yield a thin continuous layer of approximately 1/8 of an inch of thickness. There may be a mild warming sensation, or slight burning, to the treated area for 3-5 minutes after application. If irritation occurs or symptoms persist after 10 days, discontinue use and consult your physician.
Try to keep the area being treated clean and exposed to air when possible. Apply an appropriate dressing to shield the area from clothes or exposure to water or dirt.
If there is no improvement in the wound within 7 days, consult your physician for further evaluation of the wound. If there is no response to the ointment at all, then the wound should be re-evaluated for other contributing factors to the wound healing process.
- PEDIATRIC USE
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Bensal HP® inhibited all tested microbial strains, both Gram negative and Gram positive, in a Minimum Inhibitory Concentration (MIC) test against the following 49 select pathogens. Minimum Inhibitory Concentration Testing of QRB-7 The minimum inhibitory concentrations (MIC) of QRB-7 are listed below in parts per million (PPM)* . Microorganism QRB-7 - *
- Data on file: 7 Oaks Pharmaceutical Corp., Easley, SC
Microorganism Parts Per Million Staphylococcus aureus, ATCC 6538 25,000 Salmonella choleraesuis, ATCC 10708 25,000 * Enterococcus faecalis, ATCC 19433 50,000 Pseudomonas cepacia, ATCC 10856 3,125 Staphylococcus epidermidis, ATCC 17917 12,500 Alcaligenes faecalis, ATCC 8750 25,000 Streptococcus uberis ATCC 27958 12,500 Escherichia coli, ATC 25922 25,000 Klebsiella pneumoniae, ATCC 13883 25,000 Pseudomonas aeruginosa, ATCC 10145 25,000 Shigella flexneri type 1A ATTC 9199 12,500 Pseudomonas paucimobilis, ATCC 29837 1,563 Streptococcus sanguis, ATCC 10556 12,500 Acinetobacter lewoffii, ATCC 9957 25,000 Pseudomonas putida, HTB Isolate 6,250 Aeromonas sobria, ATCC 9071 25,000 Staphylococcus hominus, ATCC 27844 12,500 Staphylococcus haemolyticus, ATCC 29970 25,000 Staphylococcus saprophyticus, ATCC 15305 25,000 Staphylococcus simulans, ATCC 27848 25,000 Micrococcus lylae, ATCC 27566 50,000 Streptococcus agalactiae ATCC 13813 12,500 Streptococcus equisimilis ATCC 9542 12,500 Pseudomonas alcaligenes, ATCC 14909 25,000 Klebsiella oxytoca, ATCC 15764 12,500 Pseudomonas stutzeri, ATCC 17588 50,000 Salmonella typhi, ATCC 6539 12,500 Enterobacter aerogenes, ATCC 15038 25,000 Group D enterococcus 50,000 Trichophyton mentagrophytes CDC y68+ 50,000 Rhodotorula rubra HTB Isolate 50,000 Enterobacter cloacae, Hosp/Envi isolate 25,000 Escherichia coli, Hosp/Envi isolate 25,000 Pseudomonas cepacia, Hosp/Envi isolate 25,000 Klebsiella pneumoniae, Hosp/Envi isolate 25,000 Staphylococcus aureus, Hosp/Envi isolate 50,000 Acinetobacter calcoaceticus, ATCC 17961 25,000 Alcaligenes faecalis, ATCC 337 25,000 Enterobacter cloacae, ATCC 23355 25,000 Achromobacter xylosoxidans, HTB isolate 25,000 Salmonella typhi, ATCC 19430 25,000 Listeria monocytogenes, ATCC 15313 12,500 Serratia marcesans, ATCC 14756 25,000 Serratia marcesans, ATCC 13880 25,000 Candida albicans, ATCC 10231 12,500 Serratia marcensans, Hosp/Envi isolate 25,000 Salmonella enteritidis, ATCC 13076 25,000 Escherichia coli, ATCC 11229 25,000 Proteus mirabilis, ATCC 9240 25,000 - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 gram Tube Carton
-
INGREDIENTS AND APPEARANCE
BENSAL HP
salicylic acid ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63801-107 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) QUERCUS RUBRA BARK (UNII: X26K8566JX) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63801-107-01 1 in 1 CARTON 10/01/1998 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:63801-107-15 2 g in 1 TUBE; Type 0: Not a Combination Product 10/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 10/01/1998 Labeler - SMG Pharmaceuticals, LLC (079332298) Establishment Name Address ID/FEI Business Operations Dynamic Pharmaceuticals 617660712 MANUFACTURE(63801-107)