Label: PLUM DUO EMERGENCY EYEWASH PH NEUTRALIZING- water liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 67691-201-22 - Packager: Bel-Art Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 20, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Ask a doctor if you have
- Keep out of reach of children
-
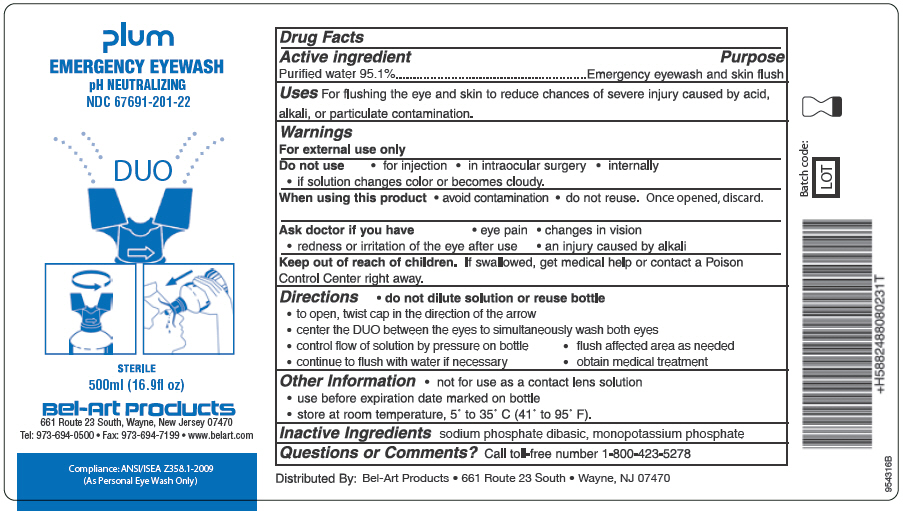
Directions
● do not dilute solution or reuse bottle
● to open, twist cap in the direction of the arrow
● center the DUO between the eyes to simultaneously wash both eyes
● control flow of solution by pressure on bottle ● flush affected area as needed
● continue to flush with water as necessary ● obtain medical treatment
- Other information
- Inactive ingredients
- QUESTIONS
- Distributed by
-
PRINCIPAL DISPLAY PANEL
Plum
EMERGENCY EYEWASH
pH NEUTRALIZING
NDC 67691-201-22
DUO
STERILE
500 ml (16.9 fl oz)
Bel-Art Products
661 Route 23 South, New Jersey, 07470
Tel: 973-694-0500 Fax: 973-694-7199 www.belart.comCompliance: ANSI/ISEA Z358.1-2009
(As a Personal Eyewash Only)Duo pH Neutralizer Label r1.jpg
Duo pH Neutralizer Label r1.jpg
-
INGREDIENTS AND APPEARANCE
PLUM DUO EMERGENCY EYEWASH PH NEUTRALIZING
water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67691-201 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) Water 95.1 mL in 100 mL Inactive Ingredients Ingredient Name Strength Sodium Phosphate, Dibasic (UNII: GR686LBA74) Potassium Phosphate, Monobasic (UNII: 4J9FJ0HL51) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67691-201-22 500 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 06/23/2010 Labeler - Bel-Art Products (002176733) Establishment Name Address ID/FEI Business Operations Plum A/S 305299489 manufacture(67691-201) Establishment Name Address ID/FEI Business Operations Holopack Verpackungstechnik GmbH 343390324 Manufacture(67691-201)