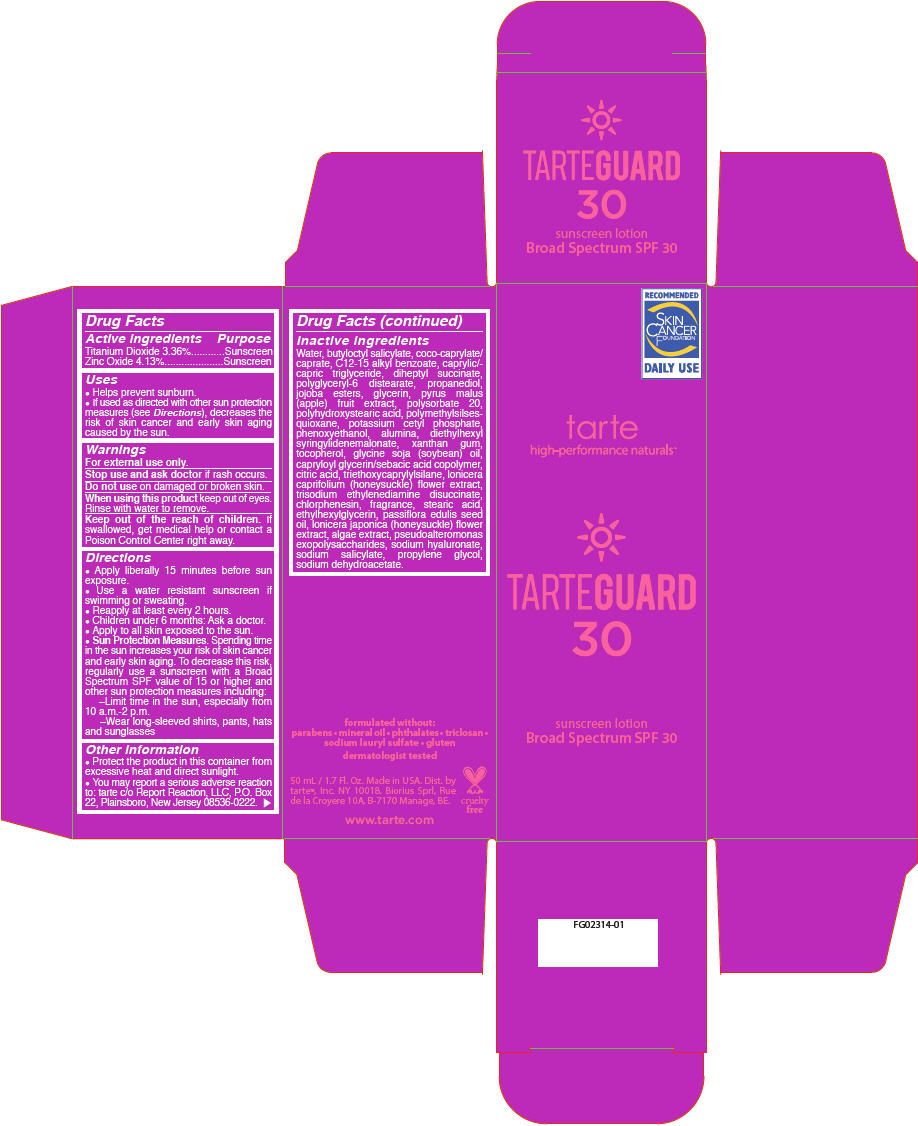
| Active ingredients | Purpose |
| Titanium Dioxide 3.36% | Sunscreen |
| Zinc Oxide 4.13% | Sunscreen |
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Stop use and ask doctor if rash occurs.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
- Apply to all skin exposed to the sun.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
Other information
- Protect the product in this container from excessive heat and direct sunlight.
- You may report a serious adverse reaction to: tarte c/o Report Reaction, LLC, P.O. Box 22, Plainsboro, New Jersey 08536-0222.
Inactive ingredients
Water, butyloctyl salicylate, coco-caprylate/caprate, C12-15 alkyl benzoate, caprylic/capric triglyceride, diheptyl succinate, polyglyceryl-6 distearate, propanediol, jojoba esters, glycerin, pyrus malus (apple) fruit extract, polysorbate 20, polyhydroxystearic acid, polymethylsilsesquioxane, potassium cetyl phosphate, phenoxyethanol, alumina, diethylhexyl syringylidenemalonate, xanthan gum, tocopherol, glycine soja (soybean) oil, capryloyl glycerin/sebacic acid copolymer, citric acid, triethoxycaprylylsilane, lonicera caprifolium (honeysuckle) flower extract, trisodium ethylenediamine disuccinate, chlorphenesin, fragrance, stearic acid, ethylhexylglycerin, passiflora edulis seed oil, lonicera japonica (honeysuckle) flower extract, algae extract, pseudoalteromonas exopolysaccharides, sodium hyaluronate, sodium salicylate, propylene glycol, sodium dehydroacetate.
Dist. by tarte™, Inc. NY 10018.
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
RECOMMENDED
SKIN
CANCER
FOUNDATION
DAILY USE
tarte
high-performance naturals™
TARTEGUARD
30
sunscreen lotion
Broad Spectrum SPF 30