ZIMS ADVANCED COLD SORE AND FEVER BLISTER MOISTURIZING PROTECTANT WITH PURIFIED PROPOLIS- petrolatum
PERFECTA PRODUCTS, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
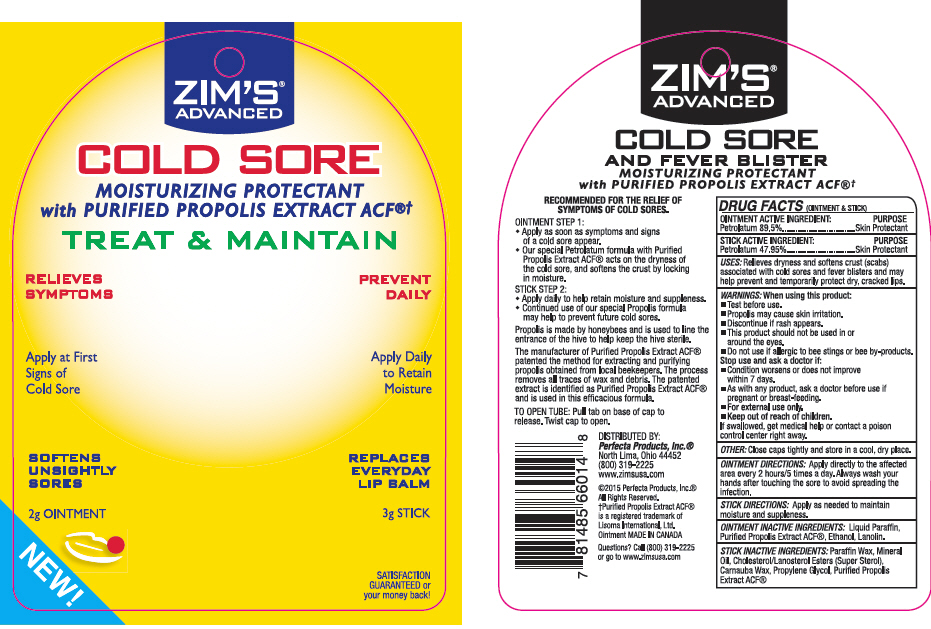
ZIMS® ADVANCED COLD SORE AND FEVER BLISTER MOISTURIZING PROTECTANT WITH PURIFIED PROPOLIS
USES
Relieves dryness and softens crust (scabs) associated with cold sores and fever blisters and may help prevent and temporarily protect dry, cracked lips.
WARNINGS
OINTMENT DIRECTIONS
Apply directly to the affected area every 2 hours/5 times a day. Always wash your hands after touching the sore to avoid spreading the infection.
STICK INACTIVE INGREDIENTS
Paraffin Wax, Mineral Oil, Cholesterol/Lanosterol Esters (Super Sterol), Carnauba Wax, Propylene Glycol, Purified Propolis Extract ACF®
DISTRIBUTED BY:
Perfecta Products, Inc.®
North Lima, Ohio 44452
(800) 319-2225
www.zimsusa.com
©2015 Perfecta Products, Inc.®
All Rights Reserved.
†Purified Propolis Extract ACF®
is a registered trademark of
Lisoma International, Ltd.
Ointment MADE IN CANADA
PRINCIPAL DISPLAY PANEL - Kit Blister Pack
ZIM'S ADVANCED
COLD SORE
AND FEVER BLISTER
MOISTURIZING PROTECTANT
with PURIFIED PROPOLIS EXTRACT ACF®†
TREAT & MAINTAIN
RELIEVES SYMPTOMS
Apply at First Signs of Cold Sore
SOFTENS UNSIGHTLY SORES
2g OINTMENT
NEW!
PREVENT DAILY
Apply Daily to Retain Moisture
REPLACES EVERYDAY LIP BALM
3g STICK
SATISFACTION GUARANTEED or your money back!
| ZIMS ADVANCED COLD SORE AND FEVER BLISTER MOISTURIZING PROTECTANT WITH PURIFIED PROPOLIS
petrolatum kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - PERFECTA PRODUCTS, INC. (868026964) |

