NEUTROGENA NATURALS ACNE SPOT TREATMENT- salicylic acid lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
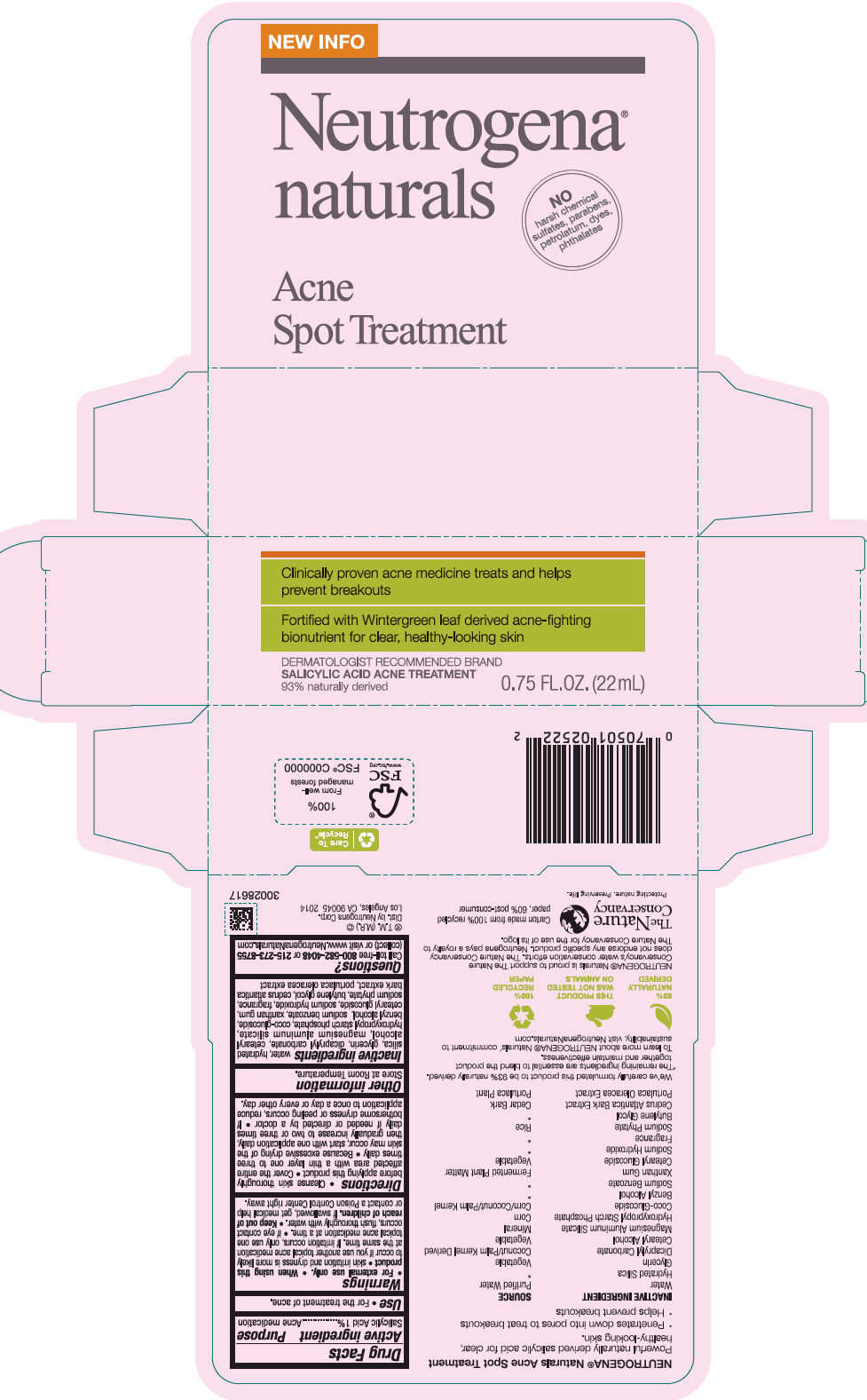
Neutrogena® Naturals Acne Spot Treatment
Warnings
For external use only.
Directions
- Cleanse skin thoroughly before applying this product
- Cover the entire affected area with a thin layer one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
water, hydrated silica, glycerin, dicaprylyl carbonate, cetearyl alcohol, magnesium aluminum silicate, hydroxypropyl starch phosphate, coco-glucoside, benzyl alcohol, sodium benzoate, xanthan gum, cetearyl glucoside, sodium hydroxide, fragrance, sodium phytate, butylene glycol, cedrus atlantica bark extract, portulaca oleracea extract
Questions?
Call toll-free 800-582-4048 or 215-273-8755 (collect) or visit www.NeutrogenaNaturals.com
PRINCIPAL DISPLAY PANEL - 22 mL Tube Carton
NEW INFO
Neutrogena®
naturals
NO
harsh chemical
sulfates, parabens,
petrochemicals,
dyes, phthalates
Acne
Spot Treatment
Clinically proven acne medicine treats and helps
prevent breakouts
Fortified with Wintergreen leaf derived acne-fighting
Bionutrient for clear, healthy-looking skin
DERMATOLOGIST RECOMMENDED BRAND
SALICYLIC ACID ACNE TREATMENT
93% naturally derived
0.75 FL.OZ. (22mL)
| NEUTROGENA NATURALS ACNE SPOT TREATMENT
salicylic acid lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |