RX ACT PAIN RELIEF 8 HR- acetaminophen tablet, film coated, extended release
H E B
----------
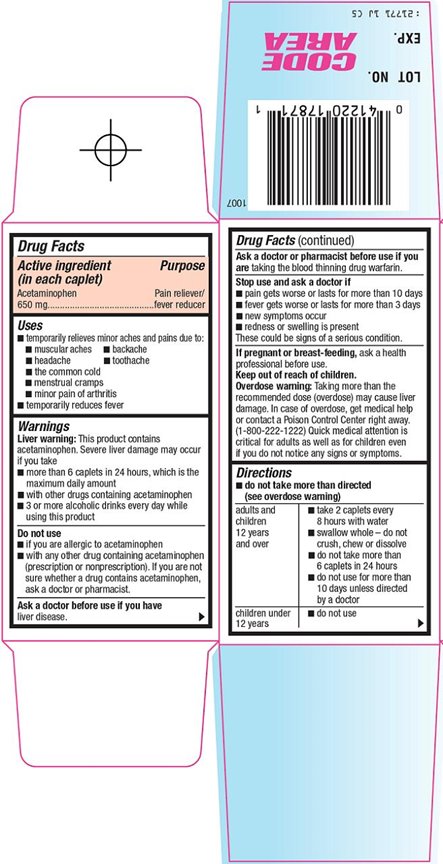
HEB Pain Relief 8 HR Drug Facts
Uses
- •
- temporarily relieves minor aches and pains due to:
- •
- muscular aches
- •
- backache
- •
- headache
- •
- toothache
- •
- the common cold
- •
- menstrual cramps
- •
- minor pain of arthritis
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 6 caplets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Do not use
- •
- if you are allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- •
- pain gets worse or lasts for more than 10 days
- •
- fever gets worse or lasts for more than 3 days
- •
- new symptoms occur
- •
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- •
- do not take more than directed (see overdose warning)
|
adults and children 12 years and over |
|
|
children under 12 years |
|
Other information
- •
- store at 20°-25°C (68°-77°F). Avoid excessive heat 40°C (104°F).
- •
- see end panel for lot number and expiration date
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, FD&C red #40 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol 400, polysorbate 80, povidone, pregelatinized starch, stearic acid, titanium dioxide
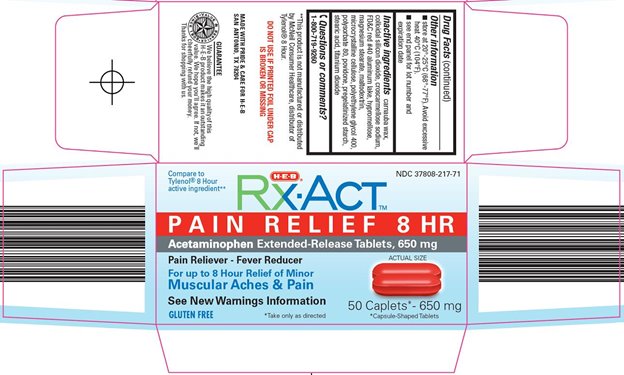
Principal Display Panel
Compare to Tylenol® 8 Hour active ingredient
PAIN RELIEF 8 HR
Acetaminophen Extended-Release Tablets, 650 mg
Pain Reliever - Fever Reducer
ACTUAL SIZE
For up to 8 Hour Relief of Minor Muscular Aches & Pain
See New Warnings Information
650 mg
GLUTEN FREE
*Take only as directed
*Capsule-Shaped Tablets
Pain Relief 8 HR Carton Image 2
| RX ACT PAIN RELIEF 8 HR
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - H E B (007924756) |