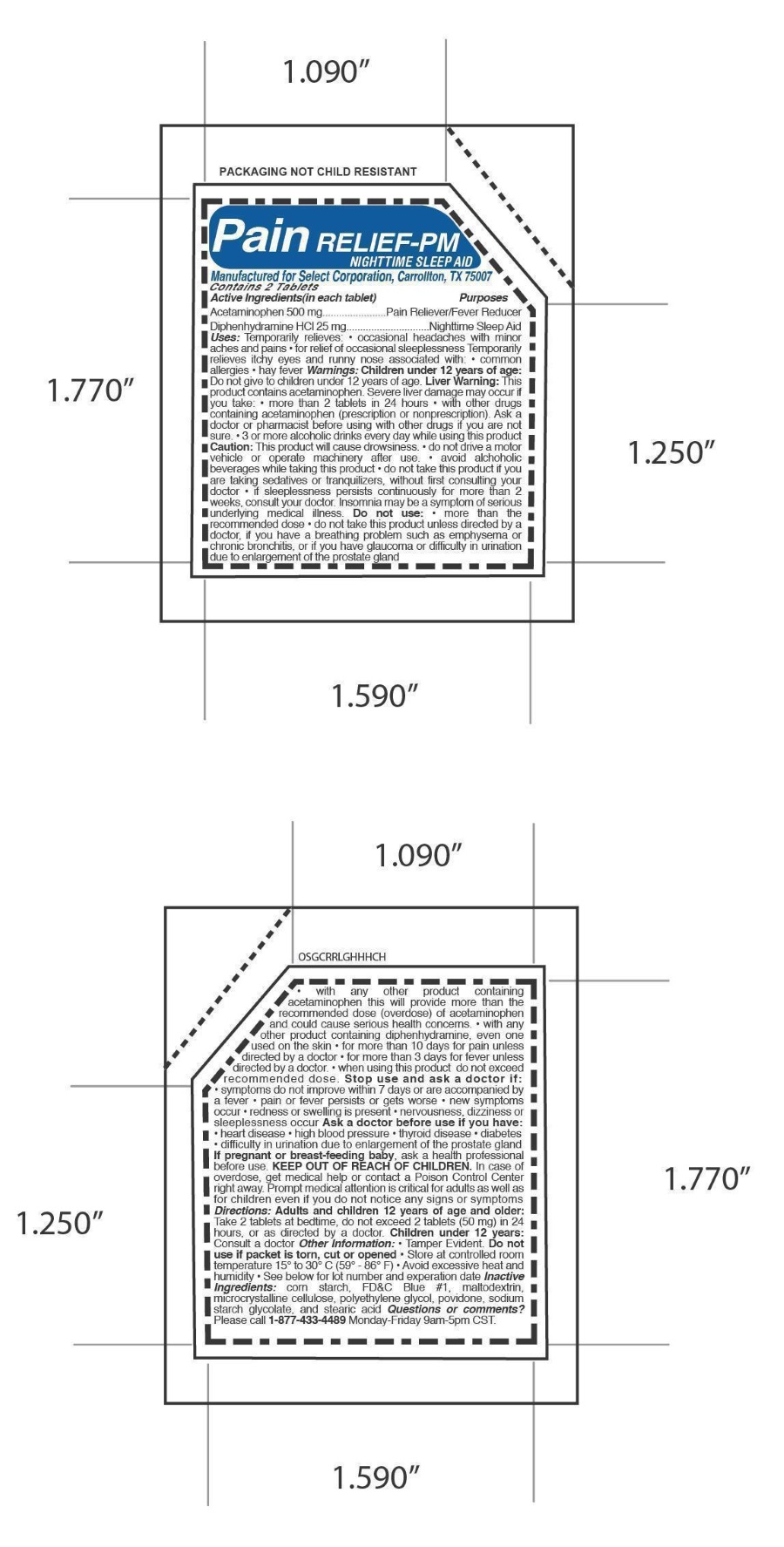
PAIN RELIEF PM NIGHTIME SLEEP AID- acetaminophen,diphenhydramine hcl tablet, coated
Select Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pain Relief PM Nightime Sleep Aid
ACTIVE INGREDIENT
Active Ingredients Purpose
Acetaminophen 500 mg.......................Pain Reliever/Fever Reducer
Diphenhydramine HCl 25 mg...............Nighttime Sleep Aid
DOSAGE
Directions: Adults and children 12 years of age and older:
Take 2 tablets at bedtime, do not exceed 2 tablets (50 mg) in 24
hours, or as directed by a doctor. Children under 12 years:
Consult a doctor
USAGE
Uses: Temporarily relieves: • occasional headaches with minor
aches and pains • for relief of occasional sleeplessness Temporarily
relieves itchy eyes and runny nose associated with: • common
allergies • hay fever Warnings:
WARNINGS
Children under 12 years of age:
Do not give to children under 12 years of age.
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if
you take: • more than 2 tablets in 24 hours • with other drugs containing acetaminophen (prescription or nonprescription). Ask a
doctor or pharmacist before using with other drugs if you are not sure. • 3 or more alcoholic drinks every day while using this product
Caution: This product will cause drowsiness. • do not drive a motor vehicle or operate machinery after use. • avoid alchoholic
beverages while taking this product • do not take this product if you are taking sedatives or tranquilizers, without first consulting your
doctor • if sleeplessness persists continuously for more than 2 weeks, consult your doctor. Insomnia may be a symptom of serious
underlying medical illness.
Do not use: • more than the recommended dose • do not take this product unless directed by a
doctor, if you have a breathing problem such as emphysema or chronic bronchitis, or if you have glaucoma or difficulty in urination
due to enlargement of the prostate glandwith any other product containing acetaminophen this will provide more than the
recommended dose (overdose) of acetaminophen and could cause serious health concerns. • with any other product containing diphenhydramine, even one used on the skin • for more than 10 days for pain unless directed by a doctor • for more than 3 days for fever unless directed by a doctor. • when using this product do not exceed recommended dose.
Stop use and ask a doctor if:
• symptoms do not improve within 7 days or are accompanied by
a fever
• pain or fever persists or gets worse • new symptoms
occur
• redness or swelling is present • nervousness, dizziness or
sleeplessness occur
Ask a doctor before use if you have:
• heart disease • high blood pressure • thyroid disease • diabetes
• difficulty in urination due to enlargement of the prostate gland.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as
for children even if you do not notice any signs or symptoms
PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding baby, ask a health professional
before use.
| PAIN RELIEF PM NIGHTIME SLEEP AID
acetaminophen,diphenhydramine hcl tablet, coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Select Corporation (053805599) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Select Corporation | 053805599 | label(52904-445) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ULTRAtab Laboratories, Inc. | 151051757 | manufacture(52904-445) | |