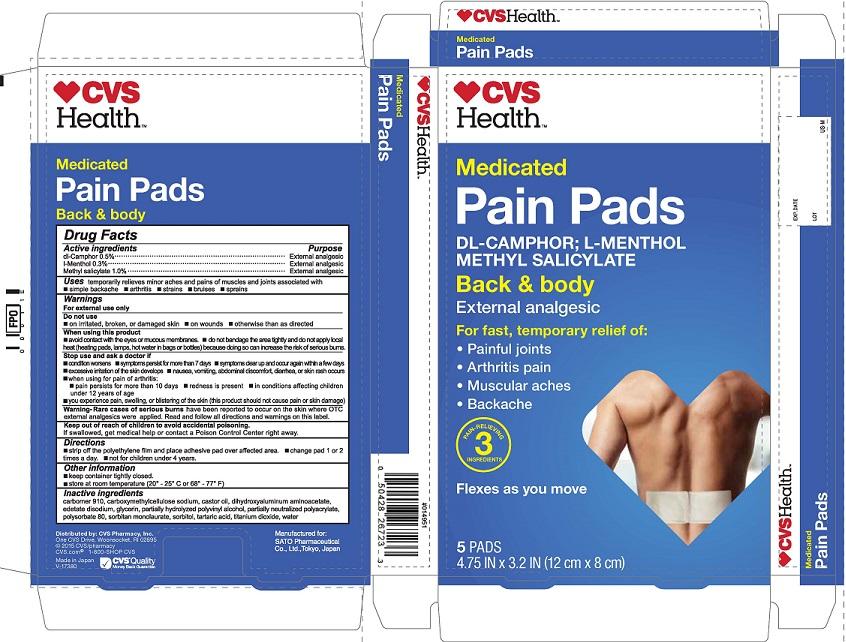
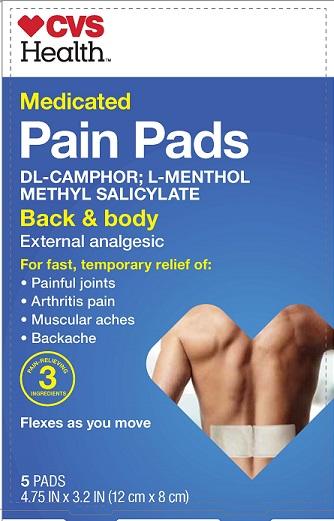
CVS MEDICATED PAIN- dl-camphor, l-menthol, methyl salicylate patch
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Purpose
dl-Camphor External analgesic
l-Menthol External analgesic
Methyl salicylate External analgesic
Uses temporarily relieves minor aches and pains of muscles and joints associated with
• simple backache • arthritis • strains • bruises • sprains
Warnings
For external use only
Do not use
• on irritated, broken, or damaged skin • on wounds • otherwise than as directed
When using this product
• avoid contact with the eyes or mucous membranes • do not bandage the area tightly and do not apply local heat (heating pads, lamps, hot water in bags or bottles) because doing so can increase the risk of serious burns
Stop use and ask a doctor if
■ condition worsens ■ symptoms persist for more than 7 days ■ symptoms clear up and occur again within a few days
■ excessive irritation of the skin develops
■ nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
■ when using for pain of arthritis:
■ pain persists for more than 10 days ■ redness is present ■ in conditions affecting children under 12 years of age
■ you experience pain, swelling, or blistering of the skin (this product should not cause pain or skin damage)
Warning-Rare cases of serious burns have been reported to occur on the skin where OTC external analgesics were applied. Read and follow all directions and warnings on this label.
Keep out of reach of children to avoid accidental poisoning.
If swallowed, get medical help or contact a Poison Control center right away.
Directions
• strip off the polyethylene film and place adhesive pad over affected area • change pad 1 or 2 times a day
• not for children under 4 years
Other information
• keep container tightly closed
• store at room temperature (20 - 25 degrees C or 68 - 77 degrees F)
Inactive ingredients
carbomer 910, carboxymethylcellulose sodium, castor oil, dihydroxyaluminum aminoacetate, edetate disodium, glycerin, partially hydrolyzed polyvinyl alcohol, partially neutralized polyacrylate, polysorbate 80, sorbitan monolaurate, sorbitol, tartaric acid, titanium dioxide, water
| CVS MEDICATED PAIN
dl-camphor, l-menthol, methyl salicylate patch |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Sato Pharmaceutical Co., Ltd. (690575642) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Oishi Koseido Co., Ltd. | 710700410 | manufacture(59779-518) , pack(59779-518) , label(59779-518) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fuji Seal, Inc. | 718023935 | pack(59779-518) , label(59779-518) | |