Label: STOKO REFRESH- triclosan liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 64064-101-01 - Packager: Evonik Stockhausen, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 21, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
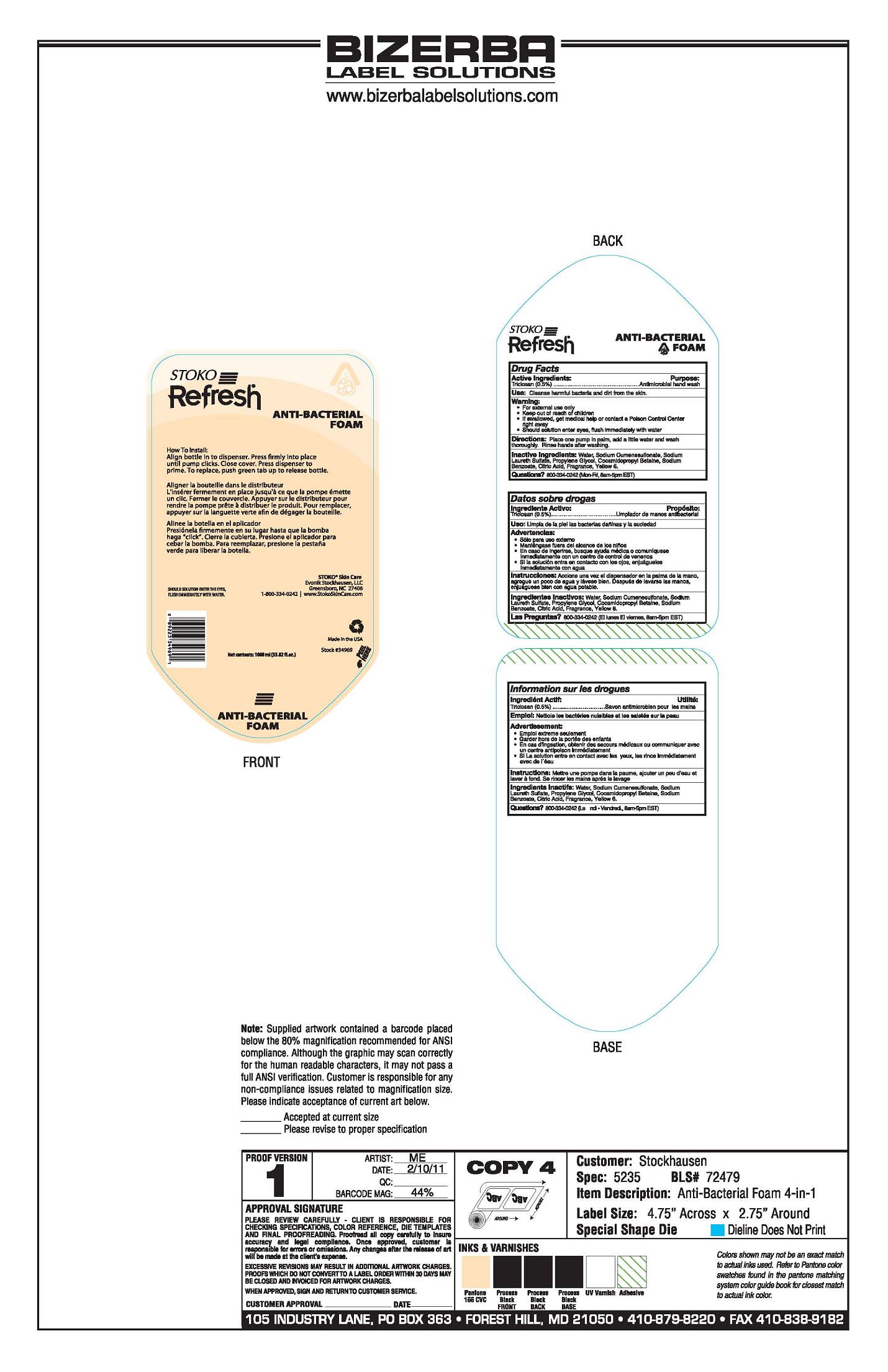
- Active Ingredients
- Purpose
- Use
- Warning
- Directions
- Inactive Ingredients
- Questions?
-
Principal Display Panel
Stoko
Refresh
Anti-Bacterial Foam
How To Install:
Align bottle in to dispenser. Press firmly into place
until pump clicks. Close cover. Press dispenser to
prime. To replace, push green tab up to release bottle.
Aligner la bouteille dans le distributeur
L'inserer fermement en place jusqu'a ce que la pompe emette
un clic. Fermer le couvercle. Appuyer sur le distributeur pour
rendre la pompe prete a distribuer le produit. Pour remplacer,
appuyer sur la languette verte afin de degager la bouteille.
Alinee la botella en el aplicador
Presionela firmemente en su lugar hasta que la bomba
haga "click". Cierre la cubierta. Presione el aplicador para
cebar la bomba. Para reemplazar, presione la pestana
verde para liberar la botella.
Should Solution Enter The Eyes
Flush Immediately With WaterStoko Skin Care
Evonik Stockhausen, LLC
Greensboro, NC 27406
1-800-334-0242 I www.StokoSkinCare.com
Made in the USA
Stock#34969
Net contents: 1000 ml (33.82 fl. oz.)
-
INGREDIENTS AND APPEARANCE
STOKO REFRESH
triclosan liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64064-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Triclosan (UNII: 4NM5039Y5X) (Triclosan - UNII:4NM5039Y5X) Triclosan 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium Cumenesulfonate (UNII: 5798KA13PG) Sodium Laureth Sulfate (UNII: BPV390UAP0) Propylene Glycol (UNII: 6DC9Q167V3) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Sodium Benzoate (UNII: OJ245FE5EU) Citric Acid Monohydrate (UNII: 2968PHW8QP) FD&C Yellow No. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64064-101-01 1000 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/01/2011 Labeler - Evonik Stockhausen, LLC (089906614) Establishment Name Address ID/FEI Business Operations Evonik Stockhausen, LLC 089906614 manufacture