SHEFFIELD BABY TEETHING GEL- benzocaine gel
Sheffield Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
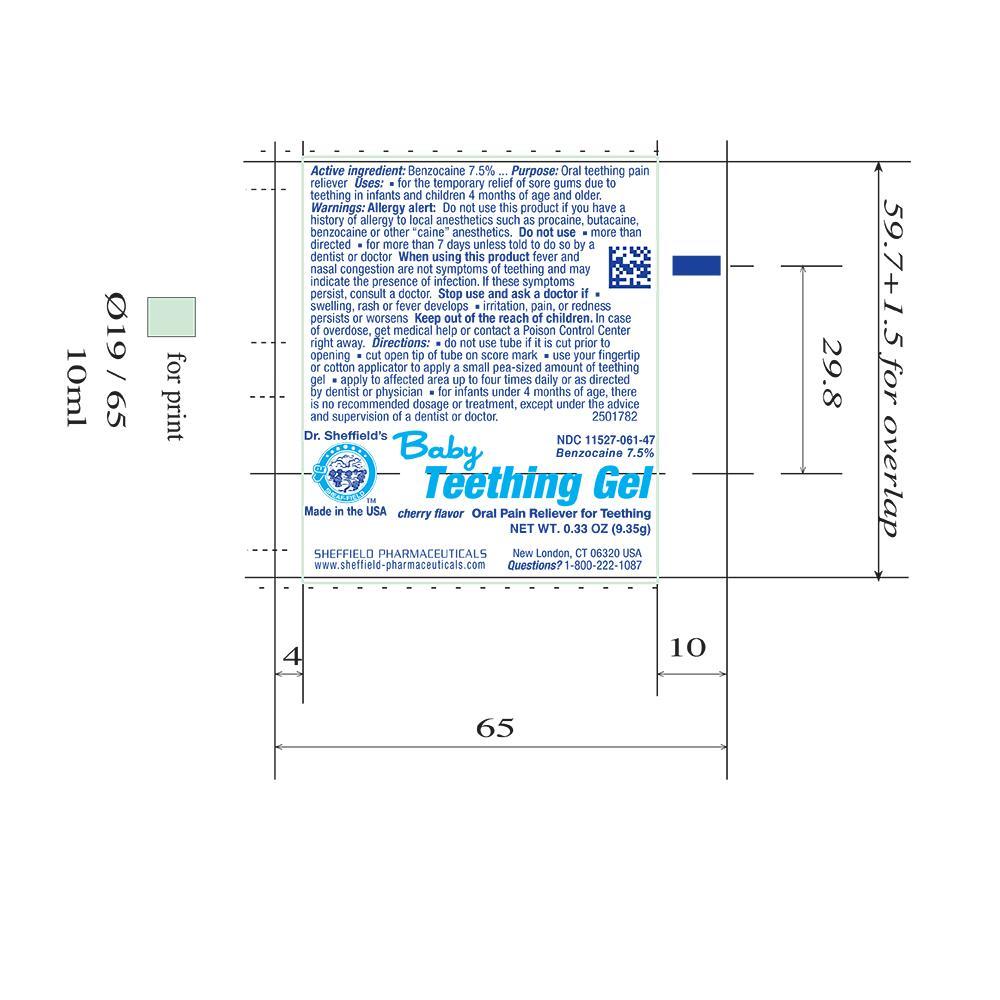
Active Ingredient
Benzocaine 7.5%
Purpose
Oral Teething Pain Reliever
Uses
- for temporary relief of sore gums due to teething in infants and children under 4 months of age and older
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Do not use
- more than directed
- for more than 7 days unless told to do so by a dentist or doctor
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms persist, consult a doctor.
Stop use and ask a doctor if
- swelling, rash or fever develops
- irritation, pain, or redness persists or worsens
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not use tube if it is cut prior to opening
- cut open tip of tube on score mark
- use your fingertip or cotton applicator to apply a small pea-size amount of teething gel
- apply to affected area up to four times daily or as directed by a dentist or physician
- for infants under 4 months of age, there is no recommended dose or treatment , except under the advice and supervision of a dentist or doctor.
Other information
- store at a controlled room temperature 59°-86°F (15°-30°C)
Inactive ingredients
D&C Yellow No. 10, FD&C Red No. 40, Flavor, Glycerin, PEG 6, PEG 8, PEG 32, PEG 75, Sodium Saccharin, Sorbic Acid, Purified water
Principal Display Panel – 0.33oz Carton Label
NDC 11527-061-47
Baby Teething Gel
Benzocaine 7.5% NET WT. 0.33 OZ (9.35g)
Oral Pain Reliever for Teething
Dr. Sheffield's
SHEAF-FIELD
Made in the USA


Principal Display Panel – 0.33oz Tube Label
NDC 11527-061-47
Baby Teething Gel
Benzocaine 7.5%
Cherry flavor Oral Pain Reliever for Teething
Dr. Sheffield's
SHEAF-FIELD
Made in the USA
NET WT. 0.33 OZ (9.35g)