CAUTION:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION:
Each ThyroMed® ChewableTablet (Levothyroxine Sodium, USP) provides synthetic crystalline levothyroxine sodium (L-thyroxine).
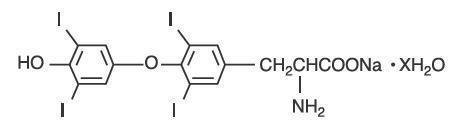
The structural formula for levothyroxine sodium is:
Levothyroxine Sodium Action
Levothyroxine sodium acts, as does endogenous thyroxine, to stimulate metabolism, growth, development and differentiation of tissues. It increases the rate of energy exchange and increases the maturation rate of the epiphyses. Levothyroxine sodium is absorbed rapidly from the gastrointestinal tract after oral administration. Following absorption, the compound becomes bound to the serum alpha globulin fraction. For purposes of comparison, 0.1 mg of levothyroxine sodium elicits a clinical response approximately equal to that produced by one grain (65 mg) of desiccated thyroid.
INDICATIONS:
Provides thyroid replacement therapy in all conditions of inadequate production of thyroid hormones. Hypothyroidism is the generalized metabolic disease resulting from deficiency of the thyroid hormones levothyroxine (T4) and liothyronine (T3). ThyroMed Chewable Tablets (levothyroxine sodium) will provide levothyroxine (T4) as a substrate for the physiologic deiodination to liothyronine (T3). Administration of levothyroxine sodium alone will result in complete physiologic thyroid replacement.
Canine hypothyroidism is usually primary, i.e., due to atrophy of the thyroid gland. In the majority of cases the atrophy is associated with lymphocytic thyroiditis and in the remainder it is non-inflammatory and as of yet unknown etiology. Less than 10 percent of cases of hypothyroidism are secondary, i.e., due to deficiency of thyroid stimulating hormone (TSH). TSH deficiency may occur as a component of congenital hypopituitarism or as an acquired disorder in adult dogs, in which case it is invariably due to the growth of a pituitary tumor.
HYPOTHYROIDISM IN THE DOG:
Hypothyroidism usually occurs in middle-aged and older dogs although the condition will sometimes be seen in younger dogs of the larger breeds. Neutered animals of either sex are also frequently affected, regardless of age. The following are clinical signs of hypothyroidism in dogs:
Dermatological
Atrophy of the epidermis, thickening of the dermis
Surface and follicular hyperkeratosis, pigmentation
Puffy face, blepharoptosis, tragic expression
Dry, coarse, sparse hair coat, slow regrowth after clipping
Retarded turnover of hair (carpet coat of boxers)
MetabolicLethargy, lack of endurance, increased sleeping
Reduced interest, alertness and excitability
Slow heart rate, weak apex beat and pulse, low voltage on ECG
Preference for warmth, low body temperature, cool skin
Increased body weight
MusculoskeletalStiff and slow movements, dragging of the front feet
Head tilt, disturbed balance, unilateral facial paralysis
ReproductiveShortening or absence of estrus, lack of libido
GastrointestinalDry feces, occasional diarrhea
Clinical pathologicalHypercholesterolemia
Normochromic, normocytic anemia
Elevated serum creatinine phosphokinase
CONTRAINDICATIONS:
Levothyroxine sodium therapy is contraindicated in thyrotoxicosis, acute myocardial infarction and uncorrected adrenal insufficiency. Use in pregnant bitches has not been evaluated.
PRECAUTIONS:
The effects of levothyroxine sodium therapy are slow in being manifested. Overdosage of any thyroid drug may produce the signs and symptoms of thyrotoxicosis including, but not limited to: polydipsia, polyuria, polyphagia, reduced heat tolerance and hyperactivity or personality change. Administer with caution to animals with clinically significant heart disease, hypertension or other complications for which a sharply increased metabolic rate might prove hazardous.
The material safety data sheet (MSDS) contains more detailed occupational safety information. For
technical service, to report an adverse event or to obtain a copy of the MSDS, call 1−800−464−3752.
KEEP OUT OF REACH OF CHILDREN
ADVERSE REACTIONS:
There are no particular adverse reactions associated with levothyroxine sodium therapy at the recommended dosage levels. Overdosage will result in the signs of thyrotoxicosis listed above under precautions.
DOSAGE:
The initial recommended dose is 0.1 mg/10 lb. (4.5 kg) body weight twice daily. Dosage is then adjusted by monitoring the thyroid blood levels of the dog every four weeks until an adequate maintenance dose is established. The usual maintenance dose is 0.1 mg/10 lb. (4.5 kg) once daily.
ADMINISTRATION:
ThyroMed® Chewable Tablets may be given orally to dogs as a treat, or crumbled over their food at the
veterinarian-prescribed dose. If crumbled over food, consumption should be monitored.
HOW SUPPLIED:
ThyroMed® Chewable Tablets are available in 0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg, 0.5 mg, 0.6 mg, 0.7
mg and 0.8 mg strength chewable tablets. Tablets are bilaterally scored. The tablets are packaged in
bottles of 180 tablets (with childproof caps) and 1,000 tablets.
STORAGE:
Store at controlled room temperature; 15° C to 30° C (59° F to 86° F) and protect from light.
Dispense product in this container or another light, light-resistant container as defined in the USP.
REFERENCES:
Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. W.B. Saunders, Co.,
Philadelphia, PA 1996: 68−117.
Scott DW, Miller WH, Griffen CE. Small Animal Dermatology. W.B. Saunders, Co., Philadelphia,
PA 1995: 237−8, 894−5.
Nelson RW. Current Veterinary Therapy X. R.W. Kirk (ed), W.B. Saunders, Co., Philadelphia, PA 1989:
993−997.
Evinger JV, Nelson RW. J Am Vet Med Assoc 1984; 185(3): 314−316.
Manufactured by Diamond Animal Health, Inc.
A wholly owned subsidiary of Heska Corporation
Des Moines, Iowa 50327
HESKA
Smarter, TogetherTM
©2011 Heska Corporation.
All Rights Reserved. HESKA and ThyroMed are registered trademarks and
Smarter, Together is a trademark of Heska Corporation in the U.S. and other countries.
02256
ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-201-11
0.1mg
(100 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-202-11
0.2mg
(200 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-203-11
0.3mg
(300 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-204-11
0.4mg
(400 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-205-11
0.5mg
(500 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-206-11
0.6mg
(600 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-207-11
0.7mg
(700 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-281-11
0.8 mg
(800 mcg)
180 Tablets

Diamond Animal Health, Inc..








