Label: AIR COMPRESSED gas
- NDC Code(s): 47632-0025-1, 47632-0025-2
- Packager: O.E. Meyer Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
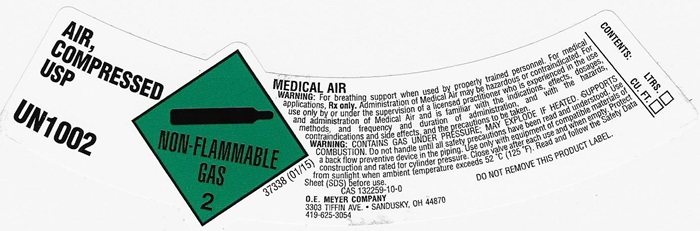
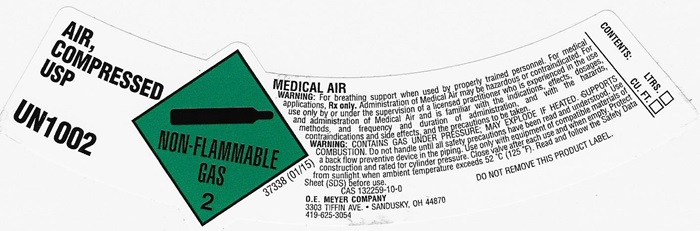
AIR, COMPRESSED LABEL
AIR, COMPRESSED USP UN1002 NON-FLAMMABLE GAS 2 37338 (01/15)
MEDICAL AIR
WARNING: For breathing support when used by properly trained personnel. For medical applications. Rx only. Administration of Medical Air may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of Medical Air and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects and the precautions to be taken.
WARNING: CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED, SUPPORTS COMBUSION. Do not handle until all safety precautions have been read and understood. Use a backflow preventive device I the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
CAS 132259-10-0 DO NOT REMOVE THIS PRODUCT LABEL.
CONTENTS: LTRS. _______
CU. FT. _______
O.E. MEYER COMPANY
3303 TIFFIN AVE. • SANDUSKY, OH 44870
419-6253054
-
INGREDIENTS AND APPEARANCE
AIR COMPRESSED
air compressed gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47632-0025 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 20 L in 100 L Inactive Ingredients Ingredient Name Strength NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47632-0025-1 5900 L in 1 CYLINDER; Type 0: Not a Combination Product 12/15/1988 2 NDC:47632-0025-2 730 L in 1 CYLINDER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 12/15/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205765 12/15/1988 Labeler - O.E. Meyer Company (555452523) Registrant - O.E. Meyer Company (555452523) Establishment Name Address ID/FEI Business Operations O.E. Meyer Co. 555452523 manufacture(47632-0025)