Label: NARAMIN- diphenhydramine hydrochloride solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 58292-010-05 - Packager: National Pharma Industries Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 28, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
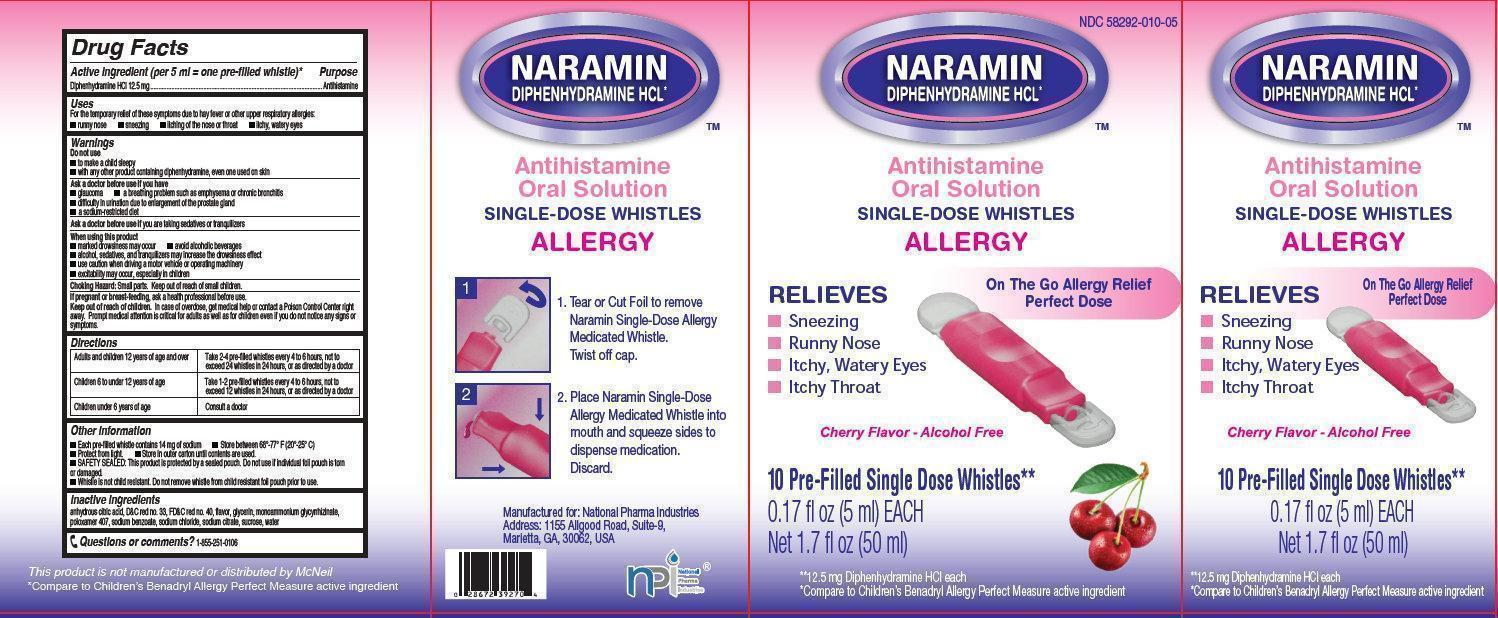
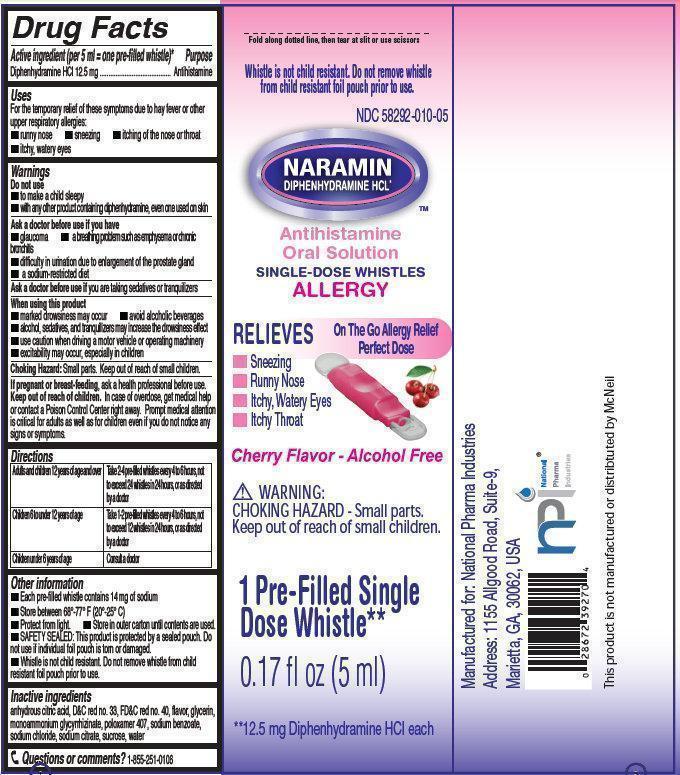
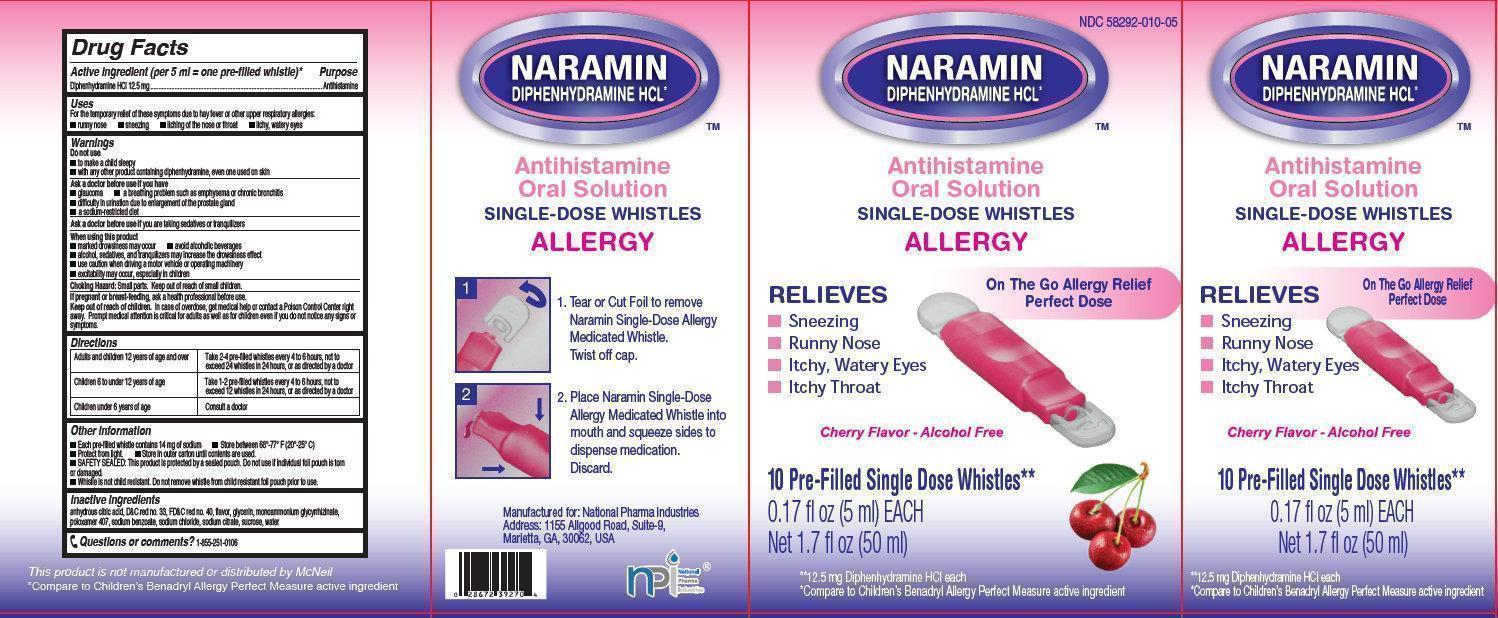
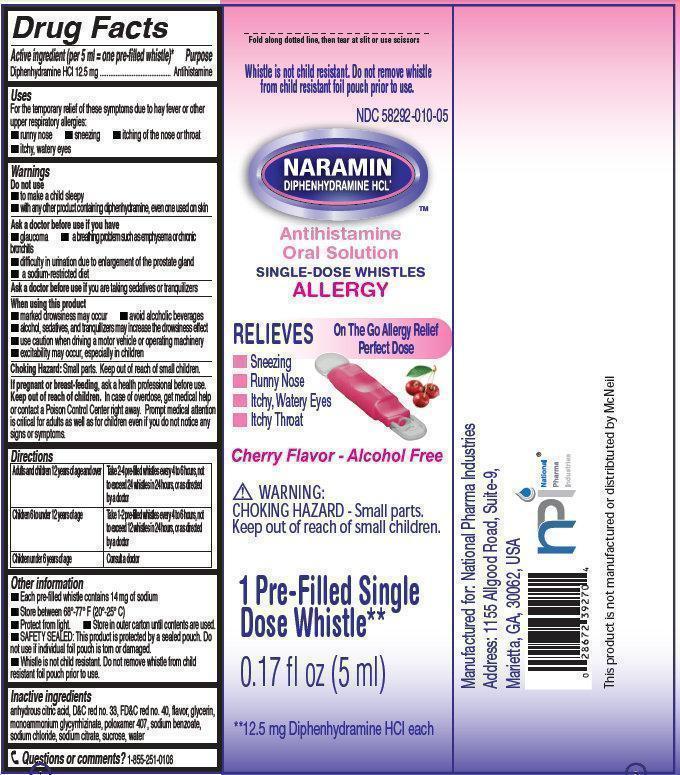
- NARAMIN Antihistamine Oral Solution
- Active ingredient (per 5 ml = one pre-filled whistle)*
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a sodium-restricted diet
- a breathing problem such as emphysema or chronic bronchitis
-
Directions
Adults and children 12 years of age and over
Take 2-4 pre-filled whistles every 4 to 6 hours, not to exceed 24 whistles in 24 hours, or as directed by a doctor
Children 6 to under 12 years of age
Take 1-2 pre-filled whistles every 4 to 6 hours, not to exceed 12 whistles in 24 hours, or as directed by a doctor
Children under 6 years of age
Consult a doctor
-
Other information
- Each pre-fille whistle contains 14 mg of sodium
- Protect from light.
- SAFETY SEALED: This product is protected by a sealed pouch. Do not use if individual foil pouch is torn or damaged.
- Whistle is not child resistant. Do not remove whistle from child resistant foil pouch prior to use.
- Store between 68 degrees-77 degrees F (20 degrees - 25 degrees C)
- Store in outer carton until contents are used.
- Inactive ingredients
- Questions or comments?
- NARAMIN Antihistamine Oral Solution 10-5ml whistles (58292-010-05)
-
INGREDIENTS AND APPEARANCE
NARAMIN
diphenhydramine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58292-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) POLOXAMER 407 (UNII: TUF2IVW3M2) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58292-010-05 10 in 1 PACKAGE 1 5 mL in 1 AMPULE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/28/2014 Labeler - National Pharma Industries Inc (078725867) Establishment Name Address ID/FEI Business Operations Plastikon Healthcare, LLC 041717941 manufacture(58292-010)